Glycation and Glycosylation 12 amazing difference you should know
Glycation and Glycosylation are essential processes that happen within our bodies, both playing a significant role in pathological and physiological conditions. Here, we explore these processes further by delving deeper into their definitions, methods, effects, implications, and strategies for controlling them. By understanding them more deeply we’ll gain greater insight into their impacts on health overall as well as ways we may mitigate any consequences they cause.
What is Glycation?
Glycation is a chemical process in which reduced sugars come together with free amino groups present on proteins, lipids, and nucleic acids to form bonds between their structures and sugar molecules. Glycation occurs naturally within our bodies but also when using high-temperature cooking techniques such as steaming.
Sugars like fructose and glucose reacting with proteins via the Maillard reaction produce what are known as advanced glycation final products (AGEs). AGEs are complex molecules capable of building up across tissues in your body over time.
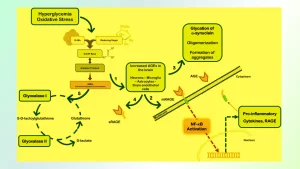
Glycation is usually an ongoing process in living organisms and foods alike. Under certain conditions like elevated blood sugar levels (hyperglycemia), this process could accelerate more rapidly; especially among diabetic patients where chronic hyperglycemia causes further development as well as accumulation of Advanced Glycation End Products.
The buildup of advanced glycation end products (AGEs) may damage biomolecules’ structure and function, with proteins affected through their process of glycation by creating cross-links to form crosslinks that alter functions or form aggregates resulting in tissue and organ aging and leading to heart diseases, diabetes complications neurodegenerative conditions or any number of related illnesses such as Alzheimer’s.
This effect of tissue or organ aging contributes significantly towards heart diseases, diabetes complications neurodegenerative conditions or any number of related conditions related to age related illnesses as AGEs accumulate over time leading to disease related conditions including heart diseases heart complications diabetes complications neurodegenerative conditions etc.
Glycation refers to the chemical reaction between proteins, reduced or unswapped sugars or lipids and proteins which leads to advanced glycation-related end products (AGEs). Glycation plays a significant role in both aging and various chronic diseases; particularly when blood sugar levels remain consistently elevated over a prolonged period.
The Process of Glycation
The process of Glycation involves a series of chemical reactions between reducing sugars and free amino groups in proteins, lipids, or nucleic acids.
This step-by-step guide covers all the aspects of Glycation:
- Presence of Reducing Sugars: Glycation requires the presence of reducing sugars. Common examples of reducing sugars include glucose, fructose, and galactose. These sugars have a reactive carbonyl group that can initiate the glycation reaction.
- Formation of Schiff Base: The first step in glycation is the reaction between the carbonyl group of a reducing sugar and the free amino group of a protein or lipid. This forms a reversible, unstable compound called a Schiff base. The reaction is a nucleophilic addition, with the amino group acting as the nucleophile and attacking the carbonyl carbon of the sugar.
- Amadori Rearrangement: The Schiff base formed in the previous step can undergo a rearrangement known as the Amadori rearrangement. This rearrangement stabilizes the product by converting the Schiff base into a more stable ketoamine compound. This ketoamine is also reversible but more resistant to hydrolysis compared to the Schiff base.
- Formation of Advanced Glycation End Products (AGEs): Over time, the ketoamine can undergo further chemical modifications, such as oxidation and dehydration. These stable and complex molecules are known as advanced glycation end-products (AGEs). AGEs can be synthesized from various sugars or protein/lipid combinations and come with varied chemical structures.
- Accumulation of AGEs: Accumulation of Aggregated Glycoproteins formed AGEs are highly stable organic molecules found throughout many tissues and biological molecules including collagen, elastin, enzymes and fats. Their accumulation may over time, with greater accumulation occurring when chronic hyperglycemia exists such as in diabetic patients.
- Biochemical Impact: Accumulating AGEs may have dramatic biochemical ramifications on biomolecules impacted. For instance, proteins exposed to AGE formation may experience cross-linking that compromises stability and performance resulting in profound structural and functional modifications. Such changes have serious ramifications on both aging processes as well as complications and diseases linked to diabetes.
Glycation should be differentiated from glycosylation by understanding their respective processes: glycosylation is an entirely enzyme reaction in which monosaccharides (sugar molecules) bonded to proteins or biomolecules using specific enzymes to form glycoconjugates; Glycation involves chemical reduction between sugar molecules and biomolecules while glycosylation involves controlled enzyme attachment of sugar molecules enzymatically to biological macromolecules.
What is Glycosylation?
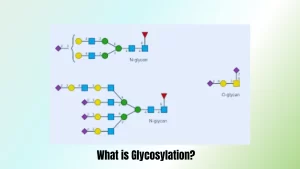
Glycosylation is an enzyme process in which monosaccharides are attached covalently to proteins or any other biomolecule through covalent bonds, making the biomolecule stable, functional and easily identifiable in cells throughout your body. Glycosylation plays an essential part of maintaining biological processes in both organisms as well as cells themselves.
Glycosylation involves specific enzymes known as glycosyltransferases which facilitate the transfer of sugar moieties from nucleotide donors onto targets biomolecules through specific acceptor sites on them, often amino acid residues or hydroxyl groups on lipids; typically connected to amino acid residues or hydroxyl groups respectively. Sugar molecules utilized vary both in terms of dimension and complexity ranging from simple monosaccharides all the way up to complex chains of oligosaccharides molecule usage during glycosylation processes.
There are two main types of glycosylation:
- N-linked glycosylation: N-linked glycosylation occurs when sugar molecules attach themselves directly to nitrogen atoms located within asparagine residues in proteins sequence. N-linked glycosylation can be found within endoplasmic-reticulum (ER), where it plays an essential role in protein folding quality control and trafficking through secretory pathways.
- O-linked glycosylation: O-linked glycosylation involves attaching sugar moieties directly to oxygen atoms present on serine (Ser) or Threonine (Thr) residues of protein chains, typically within the Golgi apparatus; further modifications can also occur adding complexity and altering chains as part of O-linked glycosylation’s role in cell adhesion, immune response and signaling processes.
Glycosylation has several essential functions in the body:
- Protein Folding and Stability: Proper glycosylation can aid in protein folding and stability, preventing misfolding and aggregation.
- Cell-Cell Recognition and Adhesion: Glycosylation plays an integral part in adhesion and cell recognition processes, including tissue development and immune response processes.
- Modulation of Protein Activity: Glycosylation as an instrument to modulate protein activity Glycosylation can have an enormous effect on proteins’ activities that determine enzyme and signal transmission pathway activities, potentially altering both enzyme activity and signal transmission paths.
- Protection from Proteolysis: Glycosylation can shield certain proteins from proteolytic degradation, extending their lifespan within the cell.
- Secretory Pathway Trafficking: Glycosylation is essential for proper protein trafficking through the secretory pathway, ensuring proteins reach their correct destinations within and outside the cell.
Glycosylation is a highly regulated and complex process, with various factors influencing the specific patterns of glycan structures attached to biomolecules. Glycosylation deficiencies or malfunction can result in numerous illnesses, from genetic glycosylation disorders (CDGs) to certain forms of cancer.
Glycosylation is an integral and complex process, playing an indispensable part in cell function development, maintaining body equilibrium and overall well-being.
The Process of Glycosylation
Glycosylation refers to an enzyme-catalyzed process of adding monosaccharides enzymatically onto proteins, fats, or other biomolecules at specific locations on them – often found on proteins, fats or biomarkers such as viral proteins – posttranslationally within cells via their endoplasmic-reticulum (ER) or Golgi apparatus in order to modify post-translationally post-translationally modified biomolecules into their final forms.
Here’s an overall overview of glycosylation:
- Sugar Activation: The first step in glycosylation is the activation of sugar molecules. Monosaccharides are activated by attaching them to a nucleotide molecule, UDP or GDP. These activated sugar nucleotides are referred to as nucleotide sugar donors.
- Sugar Transfer: The activated sugar nucleotides serve as donors of sugar moieties. Certain glycosyltransferase enzymes identify and target biomolecules (e.g. proteins or lipids) before moving sugar from nucleotide sugar sources onto an acceptor site in these targets.
- Glycosidic Linkage: When transferred between sugar molecules, an oblique bond known as a glycosidic connection forms between them and an acceptor molecule. Such acceptor molecules could include hydroxyl molecules on lipids or side chains of amino acid residues such as asparagine to promote N-linked glycosylation or serine/threonine for O-linked glycosylation on proteins.
- Further Processing in the Golgi Apparatus: After the initial sugar transfer in the ER, the glycoproteins or glycolipids are transported to the Golgi apparatus. Here, further modifications of the sugar chains can occur, involving the addition, removal, or processing of sugar residues. This leads to the generation of diverse and complex glycan structures.
- Recognition and Function: The resulting glycosylated biomolecules, now bearing specific glycan structures, play essential roles in various cellular processes. These functions include protein folding, stability, cell-cell recognition, signaling, and protection from degradation.
- Regulation: Glycosylation is a highly regulated process. The specificity of glycosyltransferase enzymes and the availability of nucleotide sugar donors determine the specific glycan structures attached to different biomolecules. These factors are influenced by various cellular and environmental signals.
Understand that the glycan structures attached to biomolecules vary according to cell type or tissue, development stage and physical conditions. Glycan structures provide another layer of control and functional diversity within biological processes in which glycosylated biomolecules participate.
Glycosylation is an intricate yet critical cellular function. Glycosylation helps maintain homeostasis and functioning; failing to regulate it could have dire repercussions for cell health and could potentially contribute to various ailments and diseases.
Comparison table of Glycation and Glycosylation
Below is a comparison table highlighting the key differences between glycation and glycosylation:
| Aspect | Glycation | Glycosylation |
|---|---|---|
| Definition | Non-enzymatic reaction between reducing sugars and free amino groups in biomolecules, forming advanced glycation end products (AGEs). | Enzymatic process where sugar molecules are covalently attached to proteins, lipids, or other biomolecules, forming glycoconjugates. |
| Type of Reaction | Non-enzymatic | Enzymatic |
| Enzyme Involvement | No enzymes required | Requires specific glycosyltransferase enzymes |
| Sugar Donor | Reducing sugars (e.g., glucose, fructose) | Nucleotide sugar donors (e.g., UDP, GDP) |
| Biomolecules Modified | Proteins, lipids, or nucleic acids | Proteins, lipids, and other biomolecules |
| Types of Glycosylation | – | N-linked (attachment to asparagine), O-linked (attachment to serine/threonine) |
| Location | Can occur both inside and outside the body | Mostly occurs within the endoplasmic reticulum and Golgi apparatus of eukaryotic cells |
| Regulation | Not regulated | Highly regulated |
| Function | Contributes to aging and age-related diseases | Plays essential roles in cellular function, protein folding, immune responses, and cell signaling |
| Biological Implications | Protein dysfunction, oxidative stress, inflammation, tissue damage | Cell recognition, protein stability, immune function, cell signaling, tissue development |
| Associated Conditions | Age-related diseases, diabetes complications | Congenital disorders of glycosylation (CDGs), cancer, immune-related disorders |
| Examples | Formation of advanced glycation end products (AGEs) | N-linked glycosylation of asparagine (Asn), O-linked glycosylation of serine (Ser) and threonine (Thr) residues |
The Role of Glycation and Glycosylation in Aging
Glycation and Glycosylation play significant roles in the aging process, albeit through different mechanisms. Both processes involve the modification of biomolecules, but they have distinct effects on cellular function and tissue integrity during aging.
Glycation and Aging
Glycation can accelerate aging through numerous processes: Production of advanced glycation end products (AGEs):
- Accumulation of AGEs: As time progresses, age-related toxins become accumulated in various organs and tissues of our body. Over time, they gather in collagen-like proteins with long lifespans; when this happens, cross-links form between proteins which lead to deformation and loss in elasticity of tissue which eventually manifests as wrinkles and changes to blood vessels, skin or other organs.
- Protein Dysfunction: Glycation can alter the structure and function of proteins, leading to impaired enzymatic activity and cellular processes. This protein dysfunction can affect various physiological functions and contribute to age-related diseases.
- Oxidative Stress: AGEs can induce oxidative stress, leading to cellular damage and dysfunction. Oxidative stress increases with age-related diseases and ailments that come with ageing.
- Inflammation: Age-related illness often coincides with chronic inflammation in the body. Chronic inflammation has long been recognized as one of the hallmarks of ageing, contributing to multiple ailments related to growing older.
- Impaired Repair and Regeneration: Glycation can interfere with tissue repair and regeneration processes, further contributing to age-related tissue deterioration.
Glycosylation and Aging
Glycosylation plays an integral part of many processes within cells and has both positive and negative implications on aging:
- Protein Folding and Quality Control: Proper glycosylation is crucial for correct protein folding and quality control. An absence of glycosylation regulation may result in proteins becoming misfolded and lead to cell dysfunction and premature aging, potentially increasing your chances of cell malfunction and age-related issues.
- Cell-Cell Communication: Glycosylation plays a key role in cell-cell communication and recognition. Changes to glycosylation patterns may influence cell signaling pathways and lead to age-related shifts in tissue homeostasis.
- Immune Function: Glycosylation is essential for immune responses and recognition of pathogens. Altered glycosylation patterns can affect immune function and increase susceptibility to infections and age-related diseases.
- Tissue Function and Regeneration: Glycosylation is involved in tissue development, maintenance, and regeneration. Changes in glycosylation can impact tissue integrity and repair processes during aging.
- Glycan Accumulation: During aging, there is a gradual accumulation of certain glycans in tissues, which can affect cellular functions and contribute to age-related changes.
Glycation and glycosylation processes are affected by both genetic and environmental influences, with their impacts on aging being both complex and interdependent. Failing to regulate these processes properly may lead to neurodegenerative conditions as well as cardiovascular or metabolic illnesses resulting in premature age-related ailments – these issues being one of many age-related ailments plaguing our population today.
Understanding the role of glycosylation and glycation during aging will allow researchers to pinpoint areas for intervention or therapies to reduce any negative consequences of these processes while simultaneously encouraging healthy ageing. Ongoing research in this area shows great promise in creating strategies to enhance health outcomes.
Managing Glycation and Glycosylation

Glycosylation and Glycation management is vital to overall health and preventing age-related illnesses.
Here are some suggestions on how you can handle these processes:
1. Diet:
- Reduce Sugar Intake: Be wary when eating foods containing refined and processed sugars; corn syrup with high fructose may reduce AGEs due to glycation reactions.
- Balanced Diet: For maximum energy and vitality, aim to consume an energizing diet comprising fruits, vegetables and whole grains as well as lean proteins and healthy fats in equal measure. This can provide essential nutrients that support proper glycosylation and overall cellular health.
2. Blood Sugar Control:
- If you have diabetes or prediabetes, managing blood sugar levels is crucial in reducing glycation reactions. Adherence to prescribed medications, insulin (if required), and lifestyle modifications can help keep blood sugar levels within a healthy range.
3. Antioxidant-Rich Foods:
- Include foods rich in antioxidants berries, leafy greens, nuts, and seeds. Antioxidants can help counteract oxidative stress induced by AGEs.
4. Regular Exercise:
- Get active regularly to increase insulin sensitivity and control blood sugar. Training has proven itself effective at increasing glycosylation and glycation processes as well.
5. Glycation Inhibitors:
- Some compounds have been identified as glycation inhibitors, which can help reduce the formation of AGEs. Examples include aminoguanidine and carnosine. As it can be beneficial to consult a medical expert prior to selecting supplements, it’s wise to seek guidance first before making decisions regarding such remedies.
6. Maintaining Healthy Weight:
- Maintaining a healthy weight is crucial for overall metabolic health and can positively impact glycation and glycosylation processes.
7. Avoid Smoking and Limit Alcohol Consumption:
- Smoking and excessive alcohol consumption can promote oxidative stress and contribute to glycation and glycosylation processes. Quitting smoking and moderating alcohol intake can be beneficial for overall health.
8. Medications and Therapies:
- Healthcare professionals may provide medication or treatments designed to manage glycosylation and glycation for specific medical conditions like diabetes.
9. Regular Health Checkups:
- Routine health checks provide a useful method for monitoring levels of blood sugar, glycosylated hemoglobin (HbA1c), and other markers related to glycocation issues allowing early identification and management.
10. Lifestyle Modifications:
- Stress management, adequate sleep, and maintaining an active and balanced lifestyle can all contribute to better overall health and help manage glycation and glycosylation processes.
It’s essential to note that while these strategies can support healthy glycation and glycosylation, complete prevention of these processes is not possible or desirable. Glycation and glycosylation are fundamental biological processes necessary for healthy cell functioning; small modifications of these processes are vitally necessary.
Our goal should be to balance this process while mitigating its negative impacts, to promote healthier aging while decreasing chances of age-related diseases. For customized advice and suggestions we always suggest consulting a healthcare provider.
Conclusion
Glycation and glycosylation are key biological processes that impact humans, both the way we age, and their quality of life. Glycation occurs as a non-enzymatic reaction between biomolecules with reduced sugars that leads to advanced glycation end products (AGEs) being formed over time, potentially disrupting protein function as well as inflammation, oxidative stress and tissue injury; additionally it plays an integral part of age related diseases including those caused by chronic hyperglycemia such as diabetes.
Glycosylation is an enzyme process in which sugar molecules bond covalently to fats, proteins and any other biomolecules through covalent bonds. Glycosylation plays an essential role in protein folding, stability and recognition by cells; its regulation must remain adequate to avoid cell dysfunction as well as diseases related to glycosylation such as cancer or genetic disorders related to glycosylation (CDGs).