What is the Difference Between Hydrocracking and Hydrotreating
Hydrotreating and hydrocracking are two vital processes within the industry of refining petroleum both of which serve distinct functions. Both require using hydrogen as well as catalysts, their purposes and methods of operation differ greatly.
This article will explore and explain the main differences between hydrocracking and treating, providing a better understanding of their processes as well as the products they use, their operating conditions, and their applications. Understanding the distinctions is vital in understanding their respective contribution to the production of fuel as well as quality improvement and environmental impacts within the refining industry.
What is Hydrocracking?
Hydrocracking is a process of refining employed in the petroleum industry to transform heavy hydrocarbons, like high-boiling point percentages of crude oil, or other feedstocks into smaller and better substances. It involves the breaking down of large, complex molecular structures into smaller and more efficient hydrocarbons at high temperatures and pressure. It also occurs with the aid of hydrogen as well as specialized catalysts.
The most important components of hydrocracking are:
Hydrogen is introduced to the process to aid in the break of chemical bonds within heavy hydrocarbons.
Catalysts and catalysts have an essential role in facilitating reactions. They are typically made of elements such as nickel, platinum, or tungsten, which are supported on an unsupported substrate, and help to facilitate the desired chemical transformations, without being consumed during the process.
High temperature and pressure hydrocracking, works at elevated temperatures (typically between 400 and 800 to 900 degrees Celsius) and at high pressures (ranging between hundreds and thousands of pounds/square inch) to trigger the required reaction of molecules and chemical rearrangements.

The main goal of hydrocracking is the production of quality lighter hydrocarbons, such as gasoline diesel, jet, and gasoline fuel from less valuable, heavier feedstocks. By breaking big hydrocarbon molecules down into smaller better-quality components, hydrocracking increases the quantity of fuels that are valuable and also improves their properties, such as the octane ratings as well as the cetane numbers.
Furthermore, hydrocracking plays a crucial role in reducing the sulfur content of fuels, thus ensuring that they comply with strict environmental regulations that aim to reduce the harmful emissions that occur when these fuels are burned.
Hydrocracking is an important refining procedure that helps satisfy the ever-growing demand for premium transportation fuels and optimizes the use of heavy crude oil components that might have less value or be difficult to refine.
What is Hydrotreating?
Hydrotreating is similar to an intensive cleaning process for crude oil or other feedstocks derived from petroleum. It involves treating these substances using hydrogen gas at moderate pressure and temperature under the supervision of special catalysts. This method specifically targets impurities such as nitrogen, sulfur as well and other contaminants within the feedstock.
Consider it purification methods using hydrogen. The introduction of the gas together with catalysts, initiates chemical reactions that break down and eliminate nitrogen, sulfur, and other unwelcome elements from hydrocarbons.
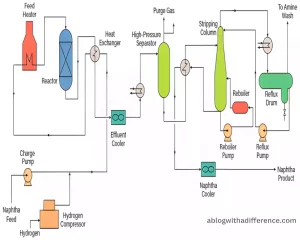
The principal objective of hydrotreating is to create cleaner and better-quality fuels by reducing sulfur content and eliminating the other impurities that cause harm. This helps ensure compliance with environmental regulations as well as enhances the overall efficiency and quality of fuels.
In essence, hydrotreating works as a refining procedure that concentrates on improving the quality of fuels by eliminating undesirable elements which makes them more environmentally sustainable and efficient in different applications, such as transportation.
Importance of these processes in the petroleum industry
Hydrocracking and hydrotreating processes play an essential part of petroleum processing and refinery operations for various reasons, and should never be underestimated as integral processes in an organization’s business operations.
1. Product Diversification: Hydrocracking enables the conversion of heavy and lower-value crude oil fractions into lighter, high-value products such as gasoline, diesel, and jet fuel. This process allows refineries to maximize the production of more valuable products from feedstocks that would otherwise be less desirable, enhancing product diversification and meeting market demands for various refined products.
2. Clean Fuel Production: Both hydrocracking and hydrotreating contribute to the production of cleaner fuels with reduced sulfur, nitrogen, and other harmful impurities. By complying with stringent environmental regulations that mandate lower sulfur content in transportation fuels, these processes aid in reducing emissions of harmful pollutants and improving air quality.
3. Upgrading Feedstocks: Hydrotreating is essential for improving the quality of crude oil or other feedstocks by removing sulfur, nitrogen, and oxygen-containing compounds. By upgrading the feedstocks, it enhances their suitability for further processing in other refining units, thereby optimizing the overall refining efficiency.
4. Environmental Compliance: As environmental concerns and regulations become more stringent, hydrocracking and hydrotreating processes are vital in reducing the environmental impact of petroleum refining. The production of cleaner fuels through these processes helps in mitigating air pollution and contributes to overall sustainability efforts.
5. Catalyst Protection: Both processes help protect downstream refining units and catalysts by removing impurities that can poison or deactivate them. This ensures the longevity and effectiveness of catalysts used in other refining processes, resulting in improved overall refinery efficiency and cost-effectiveness.
6. Petrochemical Feedstock Production: Hydrocracking is an efficient means of producing fuels for petrochemical production, an integral component of many manufactured goods such as plastics, solvents, and synthetic materials as well as lubricants and contributing significantly to the expansion of the industry.
7. Economic Benefits: By increasing the production of high-value products and complying with market demands for cleaner fuels, hydrocracking and hydrotreating processes can lead to higher refinery margins and greater profitability for petroleum companies.
8. Energy Security: These methods help maximize crude oil resources by converting feedstocks of lower value into products of greater worth, thus improving energy security by decreasing import dependence while simultaneously increasing domestic production of refined products.
The importance of hydrocracking and hydrotreating lies in their contributions to refining efficiency, cleaner fuel production, environmental compliance, and economic viability in the petroleum industry. These processes allow refineries to meet market demands, adhere to strict environmental standards, and maximize the value of their feedstocks, making them essential for the modern refining landscape.
Comparison chart of Hydrocracking and Hydrotreating
Below is a comparison chart outlining the key differences between hydrocracking and hydrotreating:
| Aspect | Hydrocracking | Hydrotreating |
|---|---|---|
| Objective | Conversion of heavy hydrocarbons into lighter, valuable products such as gasoline, diesel, and jet fuel | Removal of impurities (e.g., sulfur, nitrogen) from fuels to produce cleaner, high-quality products |
| Purpose | Upgrade heavier feedstocks into lighter, more valuable hydrocarbons | Improve fuel quality by reducing impurities for environmental compliance and enhanced performance |
| Process | Breaks down large hydrocarbon molecules by adding hydrogen and catalysts at high temperatures and pressure | Uses hydrogen and catalysts to remove sulfur, nitrogen, and other contaminants at moderate temperatures and pressure |
| Catalyst | Contains metals like platinum, nickel, or tungsten on a support structure | Often contains metals like molybdenum or cobalt on a support structure |
| Operating Conditions | High temperature (400-900°C) and pressure (hundreds to thousands of psi) | Moderate temperature (200-450°C) and pressure (tens to hundreds of psi) |
| Products | Yields lighter hydrocarbons such as gasoline, diesel, and jet fuel | Produces cleaner versions of existing fuels with reduced sulfur and nitrogen content |
| Environmental Impact | Reduces emissions by producing cleaner, higher-quality fuels | Contributes to environmental sustainability by producing fuels with reduced pollutants |
| Application | Typically used for maximizing the production of lighter, more valuable fuels | Primarily employed for compliance with environmental regulations and enhancing fuel quality |
This chart highlights the fundamental distinctions between hydrocracking and hydrotreating, emphasizing their objectives, processes, operating conditions, and the products they yield in the petroleum refining industry.
Future Trends in Hydrocracking and Hydrotreating Technologies
As the petroleum industry evolves and strives to meet growing energy demands while addressing environmental concerns, several future trends are expected in hydrocracking and hydrotreating technologies:
Advanced Catalyst Development: Research and development efforts will focus on creating more efficient and selective catalysts for both hydrocracking and hydrotreating processes. Improved catalysts will enhance product yields, increase conversion rates, and reduce energy consumption, ultimately leading to more sustainable and cost-effective operations.
Integration of Renewable Hydrogen: To reduce greenhouse gas emissions and enhance sustainability, refineries may increasingly integrate renewable hydrogen sources, such as electrolysis-powered hydrogen, into hydrocracking and hydrotreating processes. The use of renewable hydrogen can lower the carbon footprint of these processes and contribute to the overall decarbonization of the petroleum industry.
Biogenic Feedstocks: Refineries may explore using biogenic feedstocks, such as bio-oils or waste-derived hydrocarbons, in hydrocracking and hydrotreating. Integrating renewable feedstocks can offer greater feedstock flexibility and promote the production of biofuels and renewable chemicals, contributing to a more circular and bio-based economy.
Increased Focus on Hydrotreating: As environmental regulations continue to tighten, refineries may place greater emphasis on hydrotreating technologies. Hydrotreating will become crucial in producing ultra-low sulfur fuels and other environmentally friendly products, ensuring compliance with strict emissions standards.
Residue Conversion: Future hydrocracking technologies may be optimized for converting heavy residues and unconventional feedstocks, such as heavy oils, bitumen, and oil sands, into lighter, more valuable products. Innovative hydrocracking processes will be essential to unlock the full potential of these abundant but challenging feedstocks.
Hydrocracking for Chemical Production: Hydrocracking may be increasingly used to produce petrochemical feedstocks, as demand for chemicals and high-value derivatives continues to rise. Tailoring hydrocracking units for targeted chemical production can provide refineries with a competitive edge in the growing petrochemical market.
Process Intensification: Future trends in hydrocracking and hydrotreating will likely focus on process intensification, which involves increasing process efficiency, reducing energy consumption, and minimizing environmental impact. Integrating process intensification concepts, such as modular designs and enhanced heat integration, can lead to more compact and sustainable refining operations.
Digitalization and Automation: The integration of digital technologies and advanced process control systems will optimize hydrocracking and hydrotreating operations. Real-time monitoring, data analytics, and predictive maintenance will improve process reliability, reduce downtime, and enhance safety and efficiency.
Electrification: The petroleum industry may explore partial or complete electrification of certain refinery processes, including hydrogen generation and compression, to reduce reliance on fossil fuels and further decrease emissions.
Future developments of hydrocracking and hydrotreating technologies should aim to optimize efficiency while decreasing the environmental footprint, adapting to changes in energy markets, and taking into account evolving energy sector needs. Continuous innovation, research, and collaboration between refineries, research institutions, and technology providers will drive these advancements, paving the way for a more sustainable and resilient petroleum refining sector.
Conclusion
Hydrocracking and hydrotreating are essential refining processes in the petroleum industry, each serving distinct purposes and contributing to the production of high-quality fuels and valuable petrochemical feedstocks.
Hydrocracking excels in converting heavy and complex hydrocarbon feedstocks into lighter, more valuable products, such as gasoline, diesel, and jet fuel. It significantly enhances product quality, reduces sulfur content, and increases the overall yield of valuable products. However, it requires higher capital investment, operates under more severe conditions, and may lead to increased greenhouse gas emissions.
Hydrotreating, on the other hand, is an efficient means of improving feedstock’s quality by eliminating impurities like nitrogen, sulfur, and oxygen-containing materials that diminish its value. It results in cleaner, low-sulfur fuels that meet strict environmental regulations. While hydrotreating may not offer extensive product diversification like hydrocracking, it is cost-effective, environmentally beneficial, and plays a vital role in ensuring the quality of the final products.