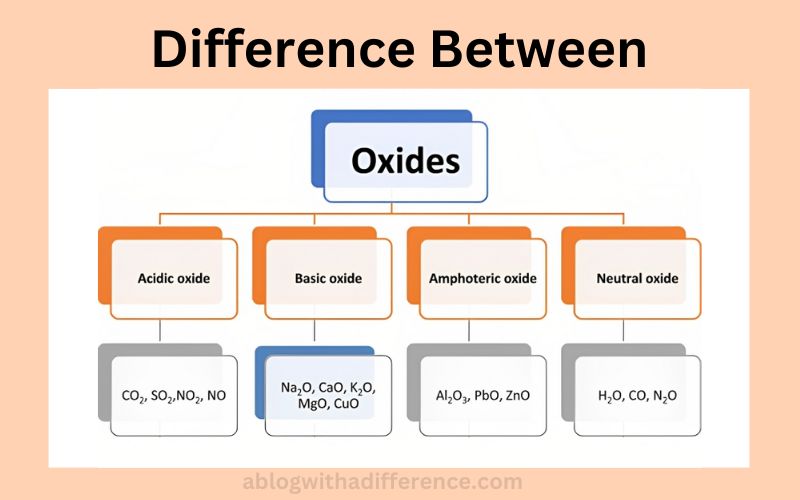
Difference Between Neutral and Amphoteric Oxides
A brief introduction to Neutral and Amphoteric Oxides
Amphoteric oxides and neutral oxides are two distinct categories of chemical compounds based on their chemical composition and properties. Neutral oxides are compounds formed through the reaction between oxygen and elements found on the periodic table, with neither showing fundamental or significant acidic characteristics when dissolved in water or reacting with bases or acids.
They are considered neutral because these reactions do not produce acidic characteristics when exposed to bases or acids, making them inert compounds that remain completely acid-neutral when encountered with acids or bases. Neutral oxides are typically composed of nonmetallic components combined with oxygen. Examples of such compounds are carbon monoxide (CO) Nitromonoxide (NO) and Water (H2O).
Amphoteric oxides, on the other hand, exhibit both acidic and basic Characteristics reacting with bases or acids under certain circumstances to form salts or other compounds depending on conditions. Amphoteric oxides are typically composed of elements with different states of oxidation that exhibit non metallic and metallic Characteristics, such as aluminum oxide (Al2O3) or zinc oxide (ZnO). Examples of Amphoteric compounds include Aluminum oxide (Al2O3) or Zinc oxide (ZnO).
Amphoteric and neutral oxides differ primarily by their response to bases and acids; neutral oxides don’t readily react with either of them and don’t show any notable acid-base interactions. Amphoteric oxides interact with acids and bases in their environment and exhibit basic or acidic properties based on conditions in their surroundings.
Amphoteric and neutral oxides have applications across a range of areas. Neutral oxides like water (H2O) are essential to life as solvents and catalysts for Biochemical reactions while amphoteric oxides such as aluminum oxide (Al2O3) and zinc oxide (ZnO) are used as catalysts abrasives ceramic pigments, or components in many products.
Understanding the physical properties and behaviors of amphoteric and neutral oxides is critical in many industrial, scientific, and environmental settings, providing us with deeper insights into chemical reactions as well as their applications.
Definition of oxides
Oxides are chemical compounds composed of oxygen atoms bonded together with various elements such as metals nonmetals or metalloids. Oxides play an integral part in many chemical processes and are often found naturally.
Oxides have different properties and reactivities depending on the elements they come into contact with and the bonding they form, including acidic, neutral, basic, or amphoteric reactions depending on their ability to react with bases or acids. Knowledge of oxide properties and attributes is vital for understanding their behavior in chemical reactions as well as applications in various fields.
Importance of understanding oxide properties
Understanding oxide properties is of significant importance for several reasons:
1. Chemical Reactions: Oxides play an integral part in chemical reactions. Their properties determine their interactions with elements like bases, acids, and other oxides; understanding these characteristics of oxides helps shape chemical reactions more precisely while aiding the creation of innovative new processes.
2. Materials Science and Engineering: Oxides play an invaluable role in material engineering and science. By understanding their properties, engineers and scientists can utilize oxides to synthesize substances with specific characteristics or modify existing oxides to meet specifications, making possible advanced materials used for applications like catalysis, electronics energy storage, or corrosion resistance. This knowledge is key to creating advanced materials used for catalysis, electronics energy storage, or corrosion resistance applications.
3. Environment Impact: Oxides can have a hugely detrimental impact on our environment, with certain oxides responsible for air pollution and smog formation. Understanding their behavior and reactivity can aid in devising plans to minimize pollution while simultaneously improving air quality; additionally, learning more about their properties helps reveal their role in natural processes like weathering and soil development.
4. Safety and Health: Oxides can pose potential threats to human health and safety. Exposure to harmful metal oxides could result in respiratory ailments and poisoning as well as other negative health consequences. Understanding their properties can help identify any risks they might present as well as implement the necessary safety precautions in industrial settings or when working with hazardous materials.
5. Industries: Oxides have many uses across a wide variety of industries. Understanding their properties is integral to optimizing industrial processes like refining, metal extraction, and fabrication; while understanding their behavior under different circumstances will increase efficiency while simultaneously decreasing costs and increasing product quality.
6. Research in Science: Oxides are an ongoing topic of scientific inquiry due to their vast properties and applications, leading to advances in fields like materials science, chemistry, and nanotechnology – this has allowed for new catalysts, materials electronic devices as well as technologies capable of revolutionizing many industries.
Understanding oxide properties is fundamental for expanding our scientific understanding, developing new materials and techniques, protecting the environment, and optimizing industrial processes – not to mention serving as a foundation for innovation and helping solve any challenges that may arise across different areas.
What exactly are neutral oxides?
Neutral oxides are chemical compounds composed of oxygen combined with elements from the periodic table, distinguished by their neutral nature that do not exhibit significant acid or base properties when dissolving in water or reacting with bases and acids.
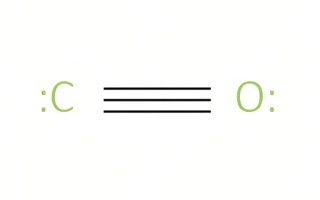
Neutral oxides typically comprise nonmetallic components combined with oxygen. They form through the oxidation process in which oxygen acquires electrons as elements lose electrons, producing what’s known as an oxide compound.
Carbon monoxide (CO), nitrogen monoxide (NO), and water are examples of neutral oxides; none exhibit distinguishable acidic or basic characteristics when they dissolve in water; carbon monoxide dissolves without either basic or acidic characteristics when dissolving in solution.
Neutral oxides play an important role in both industry and nature. Water, for instance, is indispensable to life: acting both as a solvent in biochemical reactions as well as a transport medium inside living cells. Other neutral oxides may play a part in catalysis reactions or contribute to atmospheric components.
What exactly are Amphoteric Oxides (AOs)?
Amphoteric oxides belong to a group of chemical compounds with both basic and acidic properties, making them useful in creating salts or other compounds depending on their surroundings. Their name comes from Greek words meaning both basic (amphi) and acidic (terms).
Amphoteric oxides are made up of elements with differing states of oxidation, as well as metallic and nonmetallic characteristics. As their environment dictates, amphoteric oxides can accept or release protons (H+).
One example of an amphoteric oxide can be seen in aluminum oxide (Al2O3), commonly referred to as Alumina. This substance reacts with strong acids to form salts such as aluminum chloride (AlCl3) while when combined with strong bases it can form aluminum hydroxide (Al(OH)3).
Zinc oxide (ZnO) provides another prime example. When in contact with acids, it produces salts such as zinc chloride (ZnCl2). Furthermore, when combined with bases it creates zincates such as sodium zincate (Na2ZnO2).

Amphoteric oxides have many applications; aluminum oxide is often employed as a catalyst in ceramic production as an abrasive and Refractory material, while zinc oxide can be found used as cosmetic pigments or even an ingredient of sunscreen lotions. These compounds’ amphoteric nature allows them to participate in numerous chemical reactions, making them useful in both basic and acidic environments.
Difference Between Neutral and Amphoteric Oxides
The main difference between neutral and amphoteric oxides lies in their reactivity towards acids and bases:
1. Reactivity between bases and acids:
Neutral Oxides: Neutral Oxides do not interact significantly with bases or acids in water solutions, remaining neutral at pH 7 throughout. They will therefore have little or no impact on changing its pH value and remain at 7 in solutions where they’re present.
Amphoteric oxides: Amphoteric oxides have the unique ability to react with both bases and acids; in an acidic atmosphere they act as bases by accepting protons (H+), while when in normal settings they behave more as acids by giving off protons (H+).
2. The pH Behavior:
Neutral Oxides: These oxides will not alter the pH value when they dissolve in water since they don’t produce large quantities of H+ or OH- ions.
Amphoteric Oxides: Amphoteric compounds have the unique ability to alter the pH levels in a solution depending on its basicity or acidity in response to environmental influences, showing either basic or acidic characteristics depending on how acidic or basic their surroundings are. They will often show either basic or acidic behavior depending on where their pH lies in relation to other environmental variables.
3. Examples:
Neutral Oxides: The following examples of neutral oxides are Carbon Monoxide (CO), Nitrous Oxide (N2O), and Dinitrogen Pentoxide (N2O5) are all considered neutral oxides.
Amphoteric oxides: Some well known amphoteric oxides include aluminum oxide (Al2O3) Zinc oxide (ZnO) and lead oxide (PbO).
4. Applications:
Neutral Oxides: These neutral oxygen compounds have multiple uses; carbon monoxide serves as a reducer in metallurgical processing while nitrous oxide serves as an anesthetic in medicine.
Amphoteric Oxides: Amphoteric compounds find applications in catalysis, semiconductors, and ceramics as well as in other areas where their ability to react with bases and acids provides advantages.
Amphoteric oxides differ significantly from neutral oxides due to how they react with bases and acids; neutral oxides do not react with either, while amphoteric oxides can react differently depending on pH conditions. Amphoteric oxides exhibit pH-dependent behavior, making them useful in many industries.
Significance and Applications
The understanding of the difference between neutral and amphoteric oxides has significant implications and applications in various fields:
1. Materials Science and Engineering: Understanding oxide properties such as neutrality and amphoteric properties is fundamental in creating and developing materials tailored specifically for specific purposes, such as catalysts, corrosion-resistant coatings, or electronic devices. This knowledge assists engineers in making products tailored precisely to these needs.
2. Chemical Synthesis and Reactions: Researching the reactions between amphoteric and neutral oxides is crucial to chemical synthesizing, as researchers can predict and control their actions, leading to new chemical compounds, pharmaceutical products, or functional materials being created.
3. Environmental Chemistry: Amphoteric and neutral oxides play an essential role in atmospheric chemistry as well as environmental processes, with effects ranging from altering air quality to contributing to acid rain formation; their properties help us understand their influence better and minimize these adverse environmental impacts.
4. Acid-Base Chemistry: The amphoteric nature of certain oxides is key in acid-base reactions. Amphoteric oxides can act both as acids and bases, serving to neutralize or buffer acidic or basic solutions. This characteristic can be employed effectively for many industrial and chemical processes requiring regulation of pH levels.
5. Industrial Applications: Both neutral and amphoteric oxides find widespread application across a variety of industries. Examples include:
- Neutral oxides such as carbon monoxide, nitrous oxide, and others are used extensively for chemical synthesis as well as medical processes.
- Amphoteric oxides such as zinc oxide and aluminum oxide can be found in Applications that range from catalysts semiconductors ceramics, and pigments.
6. Research and Development: The primary goal of ongoing research on amphoteric and neutral oxides is to gain an in-depth knowledge of their physical properties as well as behavior. Scientists can explore innovative applications, develop effective processes, as well as deepen our understanding of materials chemistry and catalysis.
Understanding the difference between amphoteric and neutral oxides is vitally important to many fields, such as materials science, chemistry, environmental science, industrial processes as well as technological innovations.
Knowledge helps create new materials, increase efficiency in processes, and advance technology across various areas of technology and research.
Factors Influencing Neutral and Amphoteric Properties
The neutral or amphoteric properties of oxides are influenced by several factors.
Here are some key factors that can impact whether an oxide exhibits neutral or amphoteric behavior:
1. Nature of the Element: Elements Involved The nature of elements present in oxides is of equal significance; nonmetals produce neutral oxides while those created using transition metals tend to create amphoteric ones.
2. Oxidation State of Metal: When it comes to amphoteric oxides, their behavior can depend on their metal’s oxidation state. For instance, transition metals that exhibit multiple states of oxidation tend to exhibit amphoteric properties, as their cations are capable of giving and receiving protons.
3. Electronic Structure: The chemical and electronic properties of an oxide compound can have a dramatic effect on its reactions, with amphoteric oxides featuring partially filled D orbitals within transition metal cations enabling protonation, electron transfer, or deprotonation reactions.
4. pH of the Environment: The environment pH plays a key role in how an oxide behaves, with amphoteric oxides having either basic or acidic properties depending on environmental conditions; neutral oxides however remain relatively untouched by pH changes in their surroundings.
5. Crystal Structure and Surface Chemistry: The crystal structure as well as the surface chemical composition of an oxide could influence its interactions with bases and acids. Active sites, surface defects, and surface charges could amplify either acidic or basic properties that the oxide may display, making it more amphoteric in its effects.
Temperature and pressure both have an impactful influence on oxide reactions. Pressures or temperatures that are too high alter their ability to react with bases or acids and cause changes to the amphoteric or neutral behavior of oxides.
Understanding these elements is not exclusive; multiple variables can impact an oxide’s amphoteric or neutral characteristics, making their study vital in order to anticipate and control its behavior during various chemical processes and applications.
Comparison Chart of Neutral and Amphoteric Oxides
Here’s a chart that compares the major distinctions between amphoteric and neutral oxides:
| Aspect | Neutral Oxides | Amphoteric Oxides |
|---|---|---|
| Reactivity to Acids | Do not cause significant reactions with acids. | Are able to react with acids, creating bases |
| Reactivity to Bases | Do not react significantly to bases. | Bases can react with acids and act as acids |
| pH Behavior | Does not significantly alter the pH | Alters pH, displaying basic or acidic behavior |
| Examples | Carbon Monoxide (CO) | Aluminium oxide (Al2O3), Zinc oxide (ZnO) (PbO), Lea oxide (PbO) |
| Reactivity of Water | They generally do not readily hydrolyze | It is possible to undergo hydrolysis to create bases or acids. |
| Industrial Applications | Chemical synthesis, metallurgy and so on. | Catalysts, ceramics, semiconductors, pigments, etc. |
| Environmental Impacts | Low impact on pH of the environment | Participates in acid-base reactions that affect the pH |
| The role of chemicals in Reactions | Limited participation | Active involvement in acid-base reactions |
This chart gives a brief outline of the distinctions between amphoteric oxides and neutral ones and their interactions with bases and acids pH and examples, water reaction, industrial applications environmental impacts, and their role for chemical reactions.
Conclusion
Understanding the difference between Neutral and Amphoteric Oxides can be critical in many industrial and scientific situations. Neutral oxides do not react substantially with bases or acids and maintain their neutral pH when dissolving in water, making them safe substances with numerous applications in industry such as metallurgy, chemical synthesizing, and medical processes.
Amphoteric oxides exhibit both basic and acidic properties depending on the pH of their surrounding environment, making them suitable for various applications including catalysis, semiconductors, ceramics, and environmental procedures. Their dual nature allows for control over pH as well as participation in acid-base reactions.