Difference Between Atactic Isotactic and Syndiotactic Polymer
A brief introduction to Atactic Isotactic and Syndiotactic Polymer
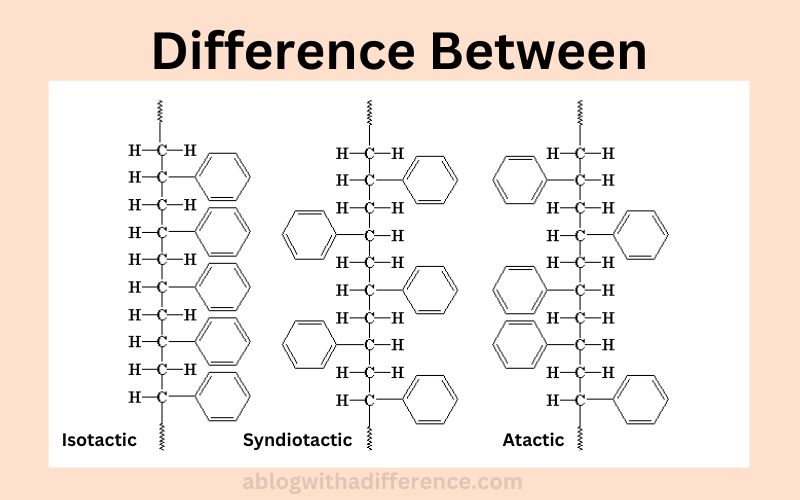
Isotactic and syndiotactic polymers differ primarily by having their substituents align in an unidirectional arrangement, while isotactic polymers may feature their substations in an identical orientation whereas syndiotactic ones contain them in a random order. Tacticity in chemistry refers to the ratio of centers that are adjacent in a macromolecule.
Macromolecules, including polymers, are large structures with many layers. Tactility plays an integral part in the determination of polymer properties as its structure regulates other aspects such as rigidity or crystallinity.
Definition of polymers
Polymers are large compounds composed of repeated monomers – subunits of molecules – chemically linked through covalent bonds to create long chains or network structures. Polymers may be natural or synthetic; natural polymers tend to have larger molecular weights and other distinctive properties than synthetic ones.
Polymers come in liquids, solids, and even gaseous states and play an integral part in many industries and applications – from fibers and plastics to adhesives, coatings, and biomaterials. Polymer properties and behaviors depend upon numerous factors, including the types of monomers used, method of polymerization, molecular structure, and arrangement of chains.
Importance of polymer structure
Polymer structure plays a key role in defining their properties, behaviors, and applications.
Here are a few points that illustrate its significance:
1. Property Identification: Polymers’ physical structures directly influence their chemical, physical mechanical and thermal properties. Molecular weight branches, crosslinking tacticity and crystallinity all impact these attributes such as strength, flexibility transparency melting point lubricity conductivity to electricity etc. By analyzing and regulating polymer structures scientists and engineers can tailor them specifically to specific uses such as medical devices.
2. Processability: The structure of polymers can have an enormous effect on their processing capability in fabrication and production, such as viscosity, flow behavior, melt temperature and extrusion process, injection molding blow molding casting, etc. A properly-designed polymer structure facilitates efficient processing for efficient product creation with top-quality results.
3. Performance Optimization: Polymers can be altered to increase their performance in certain applications, such as automotive. In particular, changes to their structure may help increase resistance to heat, impact and durability of components; and electronics use polymers with desired dielectric characteristics such as stability in temperature conditions with low water absorption for optimal insulating materials as well as circuit boards.
4. Stability and Degradability: Polymer structures play an essential role in determining their durability and degradability over time. Functional groups present within polymers as well as cross-linking density can have an effect on how resistant they are to environmental forces such as UV radiation, heat or chemicals; understanding this structure-property relationship helps design polymers with increased durability and resistance against degradation.
5. Biocompatibility and Bioactivity: When conducting biomedical research, polymer structure plays an integral part in achieving biocompatibility and bioactivity. Chemical composition and surface structure can have significant implications when interacting with living cells, tissues or biological fluids; by manipulating polymers scientists can develop biocompatible materials suitable for implant systems or drug delivery systems or scaffolds for tissue engineering applications.
6. Structure-Function Relationships: Polymers play an essential role when it comes to designing materials with specific functions. They can be engineered for stimuli-responsiveness, self-healing properties, shape memory behavior, hydrophobicity or biodegradability by altering their structure in certain ways – further increasing potential applications of these functional materials.
Polymer structures are of vital significance as they directly impact their properties, processing capabilities and overall performance as well as stability and reliability. Understanding and manipulating polymers allows us to produce materials tailored specifically to different industries and uses.
Tacticity in Polymers
Tacticity refers to the arrangement and stereochemistry of monomer units within polymers chains, particularly their arrangement in space or stereochemistry. It refers to regularity and sequence in their positioning according to stereochemistry orientation.
Tacticity plays an integral part in determining how physical and chemical properties of polymers are altered as well as crystallinity. There are three main forms of tacticity found within polymers – isotactic, atactic and syndiotactic.
1. Atactic Polymers:
- These non-stereochemically ordered chains do not adhere to an ideal stereochemical arrangement of monomer units in their chain of polymers.
- Monomer units are distributed randomly without a discernable pattern.
- Polymers classified as atractic do not exhibit crystallinity or long-range order, thus rendering them unstable.
- Atactic polymers often feature an amorphous crystal structure, leading to lower melting points and stiffer stiffness properties.
- Some examples of atactic polymers include atactic polypropylene and atactic polystyrene.
2. Isotactic Polymers:
- Its These types of polymers possess an isotactic arrangement in which substituent groups on monomer units are located to the exact side of their chains.
- Monomer units are organized and placed in an organized manner.
- Isotactic polymers have greater crystallinity than atactic ones.
- Regular configuration of isotactic polymers allows greater intermolecular interaction, leading to higher melting points and greater stiffness.
- Isotactic polymers are two of the most prevalent isotactic materials: isotactic polypropylene and isotactic polyethylene.
3. Syndiotactic Polymers:
- Syndiotactic polymers feature an unusual stereochemical arrangement in which substituent groups on monomer units switch sides within the chain.
- Monomer units should be placed in an organized, staggered arrangement.
- Syndiotactic polymers possess greater crystallinity compared to attic polymers.
- Syndiotactic polymers feature alternate groups substituting for one another to produce unique packing arrangements and properties when compared with isotactic and attic polymers.
- Syndiotactic polymers include syndiotactic styrene and syndiotactic polypropylene.
Tacticity in polymers depends on many different factors, including the stereochemistry of monomers, catalysts used for polymerization, and reaction conditions.
The tactile properties of polymers have an enormous effect on their properties, from melting point and mechanical strength to solubility and chemical reactions. By altering the tacticity of their polymer, scientists can customize its characteristics for specific uses such as elastic packaging or high-strength materials and heat-resistant ones.
Atactic Polymers
Atactic polymers are a type of polymer in which monomer units are arranged randomly across its chain of polymers without following a stereochemical arrangement pattern. The word “atactic” comes from two Greek words “a” for without and “tactic” for arrangement.
Here are the main characteristics and properties of atactic polymers:
1. Random Arranging: Atroctic polymers can be classified as atroctic when their side groups or substituents attached to their backbone can be observed on both chains without any apparent orderly pattern, creating an incoherent assortment of monomer units on either chain. This phenomenon results in chaotic configuration.
2. Amorphous Structure: Due to their random arrangement, attic polymers don’t exhibit long-range crystallinity or order; rather, they usually demonstrate an amorphous structure characterized by random polymer chains packing together without repeatable patterns.
3. Physical Properties: Attractic polymers tend to exhibit lower melting temperatures and stiffness than their syndiotactic or isotactic counterparts due to an absence of intermolecular packing that leads to weaker physical properties.
4. Solubility: Due to their amorphous properties amorphous polymers typically possess increased solubility when mixed with solvents, allowing more effective penetration of solvent Molecules into their structure resulting in greater solubility.
5. Optical Properties: The majority of atactic polymers tend to be optically inactive or exhibit lower optical activity compared to areotactic, syndiotactic or isotactic polymers due to the non-uniform arrangement of substituents that prevents light polarized by rotating in such materials.
6. Processing and Applications: Atactic polymers are frequently employed in applications requiring flexibility, low melting point and solubility – such as in elastomers, adhesives sealants coatings or modifiers used across industries like packaging construction textiles or automobile production.
Atractic polymers can be produced on purpose with specific catalysts or conditions during polymerization, or even contain regions of attic segments within otherwise syndiotactic or isotactic structures resulting in mixed or blocky tactics.
Atac polymers have distinct monomer unit arrangements which give them unique properties and applications, which make them useful in specific circumstances where their specific advantages come into play.
Isotactic Polymers
Isotactic polymers are defined as any kind of polymer in which substituent groups attached to its backbone are placed in exactly the same direction, creating a stereochemical arrangement. The term isotactic comes from Greek words meaning sameness and arrangement: iso for “same” and tactic for arrangement.
Here are a few key characteristics and features of isotactic polymers:
1. Regular Arrangement Isotactic Polymers: Here the side groups always appear in an orderly and consistent pattern across both chains, providing an orderly and consistent pattern of monomer units.
2. Crystalline Structure: The regular and well-ordered arrangement of isotactic polymers allows for increased intermolecular interaction, leading to greater crystallinity than attic polymers and producing well-defined repeat patterns within their structures that lead to various physical characteristics.
3. Higher Melting Point: Robust intermolecular forces and well-organized arrangements of isotactic polymers are responsible for higher melting points when compared with attic polymers, and allow efficient packing of polymer chains that allows more molecular cohesion.
4. Increased Physical Strength: Crystalline plastics have greater mechanical properties and stiffness due to their crystal nature and uniform packing, with strong intermolecular interactions among polymer chains facilitating efficient load transfer while resisting deformation.
5. Reduced Solubility: Isotactic polymers tend to be less soluble in solvents compared to attic polymers due to their higher level of crystallinity, as their well-organized structures impede penetration by solvents, making them less liquid.
6. Optic Properties: Isotactic polymers can exhibit optical properties due to an organized arrangement of substituent groups. They can polarize light around, making them optically active substances.
7. Applications and Processability: Isotactic polymers are known for their excellent processability, making molding easily possible into various shapes. They have applications across several industries including packaging film, fibers pipes, automotive components as well as electrical insulation applications.
Be mindful that the tactic properties of isotactic polymers can vary significantly and their degree of isotacticity (i.e. proportion of isotactic sequences in polymer chains) will influence their characteristics. Isotactic polymers may be produced using specific catalysts and controlling polymerization conditions so as to obtain stereochemical configurations which meet individual preferences.
Regular polymers feature regular crystal structures, leading to enhanced mechanical properties, greater melting points and unique optical characteristics – characteristics which make them highly sought-after across a range of situations.
Syndiotactic Polymers
Syndiotactic polymers are a type of polymer in which substituents attached to its backbone move between sides in its chain to form a particular stereochemical configuration. Syndiotactic is the term used to refer to these compounds; this word comes from Greek “syn” for together and “tactic” for arrangement.
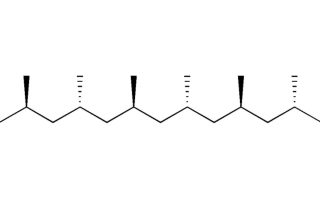
Here are the key characteristics and properties of syndiotactic polymers:
1. Alternating arrangements: Syndiotactic polymers differ from atactic and isotactic polymers by having side groups or substituents that alternate from one end of the chain to the other in a regular and repetitive fashion, making these polymers distinguishable. This alternating pattern makes syndiotactic polymers special!
2. Crystalline Structure: Syndiotactic polymers exhibit high levels of crystallinity similar to isotactic polymers, thanks to their alternated substituent groups resulting in orderly packing and strong intermolecular interactions that ultimately form distinct crystallized regions.
3. Higher Melting Point: Syndiotactic Plastics Contribute to Higher Melting Temperatures The organized and crystal structure of syndiotactic plastics contribute to higher melting temperatures than attic polymers, thanks to their organized packing that facilitates molecular cohesion as well as greater intermolecular forces.
4. Improved mechanical properties: Syndiotactic polymers usually exhibit enhanced rigidity and mechanical strength due to their crystal structure. Their regular arrangement and strong intermolecular interactions result in greater load transfer capacity as well as resistance against deformation.
5. Reduced Solubility: Syndiotactic Polymers are typically less soluble in solvents than attic polymers due to their crystallized form, and their orderly packing structure reduces penetration by solvents, thus making them less liquid-like.
6. Optic Properties: Syndiotactic polymers exhibit optical properties due to their alternating arrangement of substituted groups and can rotate in polarized light, making them optically active substances.
7. Processability and Applications: Syndiotactic polymers are often distinguished by their excellent processing capabilities, being capable of being formed into different shapes. They find use across many industries including packaging fibers, films, automobile components and special materials where their improved mechanical properties and optical characteristics are sought-after.
At all times it is crucial to keep in mind the complexity and diversity of syndiotactic polymers may vary and their characteristics could be affected by the proportion of syndiotactic sequences present within a chain of polymers. Syndiotactic polymers are created using specific catalysts with tailored conditions of polymerization in order to produce stereochemical configurations with optimal stereochemical configurations.
Syndiotactic polymers’ unique combination of alternating arrangements and crystalline structures account for their superior mechanical properties, higher melting points and optical performance – characteristics which make them highly advantageous in many applications where these features can be beneficial.
Difference Between Atactic Isotactic and Syndiotactic Polymer
Isotactic, atactic and syndiotactic polymers are distinguished from one another by their stereochemical arrangements – this determines where substituent groups appear along their chains of polymers.
Their key differences include:
1. Stereochemical Arrangemet:
- Atactic Polymers: Atactic polymers can be defined as those where substituent groups are scattered randomly within a chain of polymers; there is no specific order or pattern to their placement.
- Isotactic Polymers: These types of polymers possess an ordered and steady sequence of substituted groups on one side of their chain that are consistently spaced apart, similar to regular isotactic structures.
- Syndiotactic polymers: Exhibit an orderly arrangement of substituent groups at either end of a polymer chain, transitioning in stereoregular fashion from one end of the chain to the other in an alternating pattern.
2. Melting Points and Crystallinity:
- Atactic Polymers: These polymers typically possess an amorphous structure due to their random arrangement, leading to less crystallinity and melting.
- Isotactic Polymers: These polymers exhibit higher crystallinity due to their regular arrangement, producing well-defined crystal regions. Their melting temperatures tend to be higher compared to attic polymers.
- Syndiotactic polymers: Also boast greater crystallinity due to their regular and alternate arrangement, with higher melting temperatures than isotactic polymers.
3. Physical Properties:
- Atactic Polymers: Atactic Polymers have lower rigidity and mechanical strength compared with amorphous structures, as well as greater solubility in solvents due to their atactic structures.
- Isotactic Polymers: These materials possess greater rigidity and mechanical characteristics due to their crystal structure, yet are less soluble than attic polymers.
- Syndiotactic Polymers: Syndiotactic polymers share many of the same physical properties as isotactic polymers, yet feature improved stiffness, mechanical strength, and solubility compared to attic polymers.
4. Optics Properties of Glass Fiber Optic Cables and Hose:
- Atactic Polymers: They tend to have reduced optical activity due to a random array of substituent groups in their structure.
- Isotactic Polymers: These polymers may exhibit optical properties due to the regular distribution of substitute groups on one side.
- Syndiotactic Polymers: Exhibit optical properties thanks to their regular arrangement and an alternate pattern that substitutes groups.
5. Applications: Aseptic Polymers:
- Atactic Polymers: Aseptic polymers have numerous applications requiring their flexibility, low melting point, and solubility such as adhesives, elastomers, coatings etc.
- Isotactic Polymers: These specialized plastics are widely utilized in industries requiring exceptional mechanical stiffness, strength and thermal durability – such as in packaging fibers or automotive components.
- Syndiotactic Polymers: Syndiotactic polymers can be used in much the same way as isotactic polymers, especially where enhanced crystallinity or mechanical properties are desired.
Understanding the differences between isotactic and syndiotactic polymers lies at their core stereochemical arrangement that results in distinct chemical and physical characteristics that allow tailoring properties of polymers for specific uses.
Comparison Chart of Atactic Isotactic and Syndiotactic Polymer
This chart compares the major differences between attic syndiotactic, isotactic and syndiotact polymers:
| Aspect | Aactic Polymer | Isotactic Polymer | Syndiotactic Polymer |
|---|---|---|---|
| Stereochemical The Arrangement | A random array of substituent groupings on the chain of polymers. | The regular arrangement of the substituent group that are on one side of the chain. | The regularity of the arrangement and placement of substituents which change between the sides in the chain of polymers. |
| Crystallinity | Amorphous structure that has lower levels of crystallinity. | Crystallinity higher. | Crystallinity higher. |
| Melting Point | Lower melting point in comparison to syndiotactic and isotactic polymers. | A higher melting point than polymers with an atactic structure. | More melting points than polymers that are attic. |
| Mechanical Properties | Mechanical strength and stiffness are lower. | Increased mechanical strength and stiffness. | Stiffness and mechanical strength are increased. |
| Solubility | Higher solubility in solvents because of an amorphous structure. | Lower solubility than polymers that are atractic. | Lower solubility than polymers with an atactic nature. |
| Properties of optical Properties | Optically inactive, or with decreased optical activity. | The optical activity can be due to the regular arrangement. | The ability to detect optical activity is because of the alternating arrangement. |
| Applications | Adhesives, elastomers and coatings that require flexibility and solubility. | Fibers, packaging automotive components, and packaging materials that need rigidity and strength. | Fibers, packaging, automotive components that require rigidity and strength. |
Conclusion
The distinctions among Atactic Isotactic and Syndiotactic Polymer lie in their stereochemical configuration of substituents within their polymer chains. Atactic polymers feature irregular arrangements; while isotactic ones boast regular order on either side. Finally, syndiotactic possesses an orderly configuration that varies between sides.
Atactic polymers exhibit different properties due to the arrangement of their molecules. Atactic polymers tend to be amorphous with lower melting points and exhibit reduced mechanical strength.