Overview and key differences 1 Butene and 2 Butene
The most significant difference between 1 Butene and 2 Butene is that 1 butene has a double bond with carbon atoms located at the bottom of the chain while 2-butene is an unidirectional bond between carbon atoms located in the mid-point of the compound.
Butene is an organic chemical with its chemical formula C4H8. “Butylene” is a synonym for the exact compound. The compound is composed of four carbon atoms and eight hydrogen atoms. The compound has a double bond that connects two carbon molecules. Thus, it’s unsaturated. It is a part of the class of alkenes. It’s a non-colorless gas that is dissolved at room temperature and pressure. It is found as a minor component of crude oil. Therefore, we can acquire this substance through the catalytic process within a refinery.
Because of a double bond, the compound is a homologous one. There are four main isomers. They are But-1-ene (2Z)-but-2-ene, (2E)-but-2-ene and 2-methylprop-1-ene (isobutylene). Each of these isomers exists as a gasses. They can be liquefied by two ways: either decrease the temperature or increase the pressure. The gases emit distinct smells.
Additionally, they are extremely flammable. Due to the double bond, the compounds are less reactive than the alkanes having the same number of carbon atoms. In the context of applications for this compound, we could utilize them as monomers in the manufacture of polymers to make synthetic rubber, for the manufacturing of HDPE as well as LLDPE and LLDPE, among others.
What is 1-Butene?
1-butene is a chemical compound with its chemical formula as CH3CH2CH=CH2. It is also referred to as 1-butylene. It is a colorless gas that can be transformed into the form of a colorless liquid. It is possible to classify this chemical as an alpha-olefin linear.
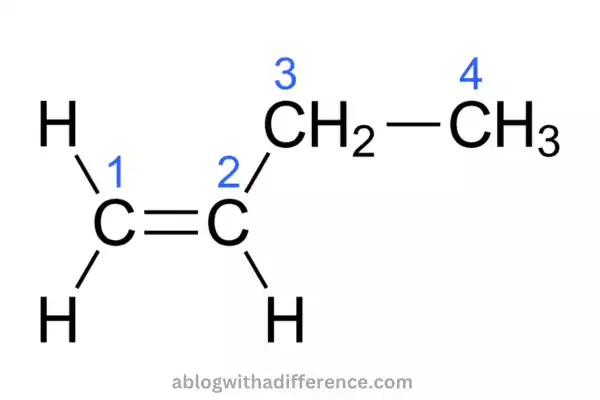
We can make 1-butene through the separation of crude C4 refinery streams as well as by the process of ethylene dimerization. Removal from the C4 refinery results in an amalgamation of 2- and 1–butane compounds. The process of ethylene dimerization produces just the alkene that is terminal.
The product can be distilled and generated by these methods to produce a pure product. Around 12 billion kilograms (1-butene) were manufactured in 2011.
What is 2-Butene?
2-butene, an organic compound that has the chemical formula CH3CH=CHCH3. It’s an alkene acyclic that has the presence of four carbon atoms. We could classify it as the most simple alkene with trans-cis isomerism. Also, 2-butene is found in two isomers, namely the trans isomer as well as the cis isomer. They are known as trans-2-butene and cis-2-butene.
2-butene is a chemical compound made by catalytic cracking of crude oil. Additionally, we can make it by dimerizing the ethylene. It is generally difficult to differentiate the two 2-butene isomers via distillation. It is due to their proximity to their boiling points. isomers.
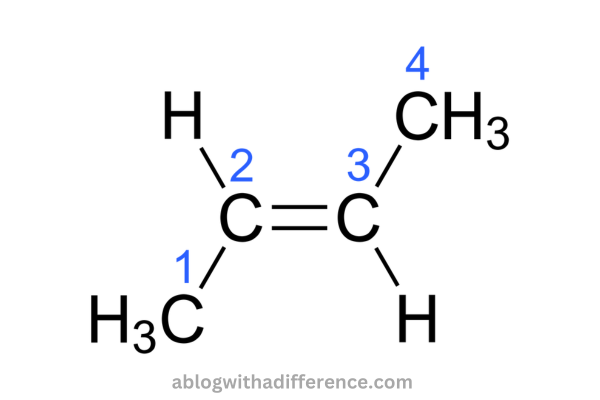
There are a variety of uses for 2-butene. They include the manufacture of butadiene and gasoline as well as making solvent butanone via the hydration process to 2-butanol, followed by an oxidation process, etc. In the majority of industrial processes, it is not necessary to separate two different isomeric forms from one the other is not necessary since each isomer behaves similarly during intended reactions.
Difference Between 1 Butene and 2 Butene
Physical Properties
The physical properties of 1-butene and 2-butene are determined by where their double bonds lie within their molecules.
- Melting Point: 1-butene has an approximate melting point of -185.35degC while 2-butene‘s melting point stands at -138.9degC; this variation in melting points owing to differences in their respective molecules’ structures is due to differences between them.
- Boiling Point: 1-butene has an approximate boiling point of -6.3degC while 2-butene’s boiling point stands at 3.7degC due to the position of its double bond, which affects intermolecular forces between molecules.
- Density: 1-butene has an approximate density of 0.614 grams/cm3, while 2-butene’s density also falls at this figure due to similar molecular weight and structure.
- Solubility: Both 1-butene and 2-butene are insoluble in water but soluble in organic solvents like ethanol or ether.
- Vapor Pressure: At 25degC, 1-butene has a vapor pressure of 285.4mmHg while 2-butene stands at 271.4 mmHg due to differences in boiling points and intermolecular forces between its molecules.
1-butene and 2-butene possess similar physical properties; however, depending on where their double bond resides can lead to some distinction in physical behavior.
Chemical Properties
The location of double bonds affects both 1-butene and 2-butene chemical properties, both positively and negatively.
- Reactivity: Both 1 and 2-butene are highly reactive molecules due to the double bond present, as evidenced by additional reactions with electrophilic compounds like hydrogen halides or water. Their position can impact their reaction’s regioselectivity; hence the products formed can differ depending on where either double bond is found.
- Stability: The position of the double bond has an effect on their respective molecules’ stabilities; typically 1-butene is more stable due to having its double bond located closer to one end, thereby being less vulnerable to isomerization or other reactions which break its double bond and isomerize into isomers or areomerizates.
- Polymerization: Both 1-butene and 2-butene can be polymerized together into polybutene for use in plastics production and other material fabrication, with different effects depending on which double bond position has been utilized to form polybutene chains resulting from its polymerization – such as molecular weight or branching properties of its final polymer product.
- Oxidation: Both 1-butene and 2-butene can undergo oxidation reactions that produce various products; such as butanal or butanoic acid. The position of double bonds affects their reaction selectivity; different products could form depending on where their double bonds lie.
1-butene and 2-butene share many similar chemical properties; however, their location of double bonds can significantly influence the reactivity, stability, and selectivity of reactions that these molecules undergo.
Production and Uses
Production:
1-Butene and 2-butene are produced through catalytic cracking of petroleum-based hydrocarbons, an industrial process that breaks large molecules of hydrocarbon down into their component molecules for purification purposes. Once created, butenes may further undergo processing steps to remove impurities and increase purity levels.
Uses:
Production of Polyethylene: Butenes play a primary role in producing polyethylene plastic used widely across a range of products ranging from packaging, films, and coatings. They serve as co-monomers in polymerization processes for various forms of ethylene polymers used as building blocks in producing numerous varieties of polyethylene with unique characteristics.
Production of Butadiene: Butenes are used in the manufacturing process of butadiene, an essential ingredient used for synthetic rubber production. Butadiene can be created via the oxidative dehydrogenation of butenes.
Butenes have multiple uses in fuel and chemicals production; from increasing octane ratings in gasoline to being converted to butanols and butadiene derivatives for use in solvent production or coating/adhesive production and other uses such as adhesives/coatings etc.
Refrigeration Applications of Butenes: Butenes can also be used as refrigerants in air conditioning and refrigeration systems, providing cooling.
Pharmaceutical Industry: Butenes play an essential part in the creation of pharmaceutical products and other fine chemicals.
Butenes play an indispensable part in producing various materials and chemicals used across numerous industries – making them an integral component of modern society.
Conclusion
1-butene and 2-butene are two important hydrocarbon molecules with distinct double bonds located at various positions on their molecules’ structures, leading to various physical, chemical, and health-related differences that impact physical, chemical, and health properties, uses, impacts on environment/health as well as use potential environmental/health applications/impacts of each hydrocarbon molecule.
1-butene and 2-butene are produced through catalytic cracking of petroleum-based hydrocarbons and are employed across an array of industries and applications, from polyethylene production and synthetic rubber synthesis to refrigerant manufacturing and pharmaceutical formulation.
Their production and use may also have harmful consequences on both human health and the environment, including air and water pollution, respiratory irritation, and even cancer risk. Therefore it is vital that butenes be handled safely so as to lessen any negative environmental consequences from release into the environment and minimize their potential repercussions.
Studying and understanding the properties and impacts of butenes are paramount to their safe use in modern societies.