Difference Between Sulfonate and Sulfate
An Introduction to Sulfonate and Sulfate
Chemical compounds with sulfur as an element have two forms. Although both appear similar at first glance, there are distinct distinctions that separate the two from one another. Sulfonate is a functional anion or group made up of sulfur bound to three oxygen molecules and one carbon atom, often represented as R-SO3-. Sulfonates are known for their strong acidity; therefore they often find employment as detergents, surfactants, and emulsifiers due to excellent water solubility as well as stability across varying pH ranges.
Contrasting with that definition is “sulfate”, an anion or functional group made up of an anionic sulfur-oxygen bond between four oxygen atoms bonded with four sulfur atoms forming SO42-. Sulfates occur naturally and play an integral part in biological processes while they have extensive uses in industrial settings like detergent production, fertilization processes, and construction materials manufacturing.
Sulfonates, which typically appear as chemical compounds, may occasionally be found in certain species of organisms and even the environment. Sulfates on the other hand are abundant throughout nature – discovered in soil, minerals, and water bodies as well as living organisms.
Sulfonates and Sulfates each serve distinct commercial and industrial functions. Sulfonates can be found widely used in personal care products and pharmaceuticals; textile processing; oilfield chemicals, as well as oilfield processing chemicals. Sulfates have agricultural uses; they’re applied as fertilizers in textile manufacture; they use them when creating paper; dye or used as additives in various products as an additive.
From both an environmental and health perspective, sulfonates and sulfates present distinct considerations. Sulfonates tend to biodegrade quickly without toxic effects if properly managed; their possible impact on aquatic ecosystems should also be carefully tracked. Sulfates occur naturally and are considered safe at normal levels; however excessive levels can have laxative-type side effects for some people.
Sulfonate and sulfate compounds each have unique characteristics that impact their structure, properties, and applications, so understanding them is of immense value in various fields such as industry, chemistry and environmental sciences.
Importance of understanding the difference between Sulfonate and Sulfate
Understanding the difference between Sulfonate and Sulfate is important for several reasons:
1. Chemical Identification: Both Sulfonates and Sulfates possess distinct chemical structures and functional groups, so distinguishing between the two compounds is key for accurate chemical analyses and identification – knowledge which proves invaluable in areas like biochemistry, organic chemistry and environmental science.
2. Industries: Both sulfonates and sulfates play important roles in industrial processes. Sulfonates can serve as surfactants, detergents and emulsifiers in personal care products and pharmaceutical processes while sulfates are utilized in detergent manufacturing, fertilizers manufacturing and construction material making processes more efficient. Knowing their differences helps determine the appropriate compound for specific applications thereby optimizing industrial processes.
3. Environment Impact: Sulfonates and sulfates both have numerous environmental ramifications. Sulfonates tend to be Biodegradable and less toxic compared to other surfactants however, their continued presence must be evaluated carefully so as to limit environmental harm. Sulfates occur naturally worldwide – understanding their behavior sources as well as the potential impact on ecosystems as well as water quality is key in order to manage or minimize their negative ramifications effectively and mitigate them effectively.
4. Health and Safety Concerns of Sulfonates/Sulfates: Sulfonates and sulfates may have various harmful impacts on human health and security, with certain varieties leading to irritation of eyes or skin and eye irritation while others could prove safer in use for various applications. Consuming large quantities through drinking water might even produce laxative effects for some individuals; so being aware of potential hazard/risk concerns associated with sulfonates/sulfates will allow appropriate handling, use, and regulations designed to safeguarding humans’ well being and health security.
5. Biological Significance: Sulfonates and sulfates play key roles in biological systems. Sulfates play an integral part in processes including protein synthesis, cell metabolism and creating connective tissues; Sulfonates may not be present everywhere within organisms yet still perform certain roles within them – understanding these differences helps researchers better comprehend these biological processes as well as understand and explore them across species.
Know the differences between Sulfonate and Sulfate chemical compounds to accurately identify applications within industry settings, environmental management issues and biological research systems. It also promotes informed decision-making to enable safe utilization across different areas.
What exactly is Sulfonate?
Sulfonates, also referred to as R-SO 3 -, are anionic compounds with chemical formula R-SO 3- that originates in Sulfonic Acid. Most often they’re stable in water solutions while remaining transparent – never being subject to oxidization! Their chemical structures include as follows.
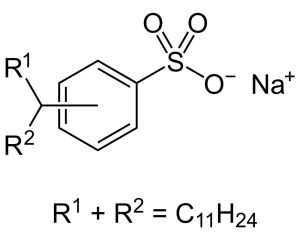
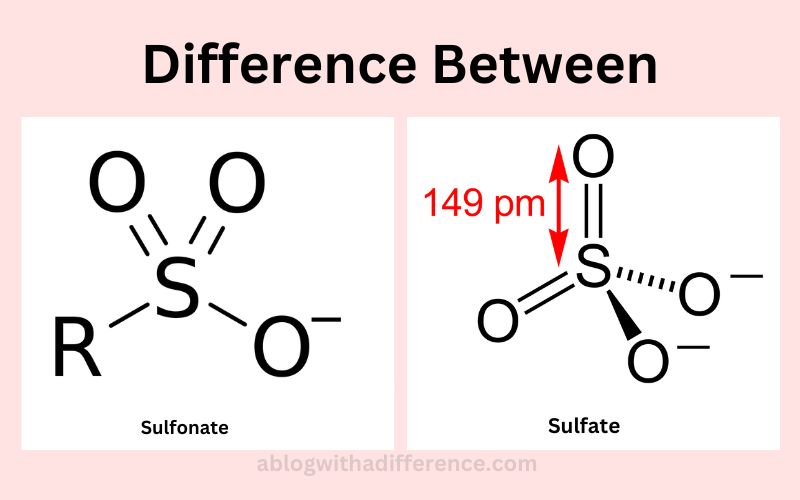
Sulfonic acids are potency acids. Since sulfonate is their conjugate base, and hence weak in comparison. Oxygenation State Value (+5) of sulfur Atom: Total Charge 1*Like the Sulfate anion, Sulfonate anion also features R-S bonds as singular bonds as well as SO double bonds; these also contribute towards its charge 1 character.
What exactly is Sulfate?
Sulfate (SO 42), produced from sulfuric acid, has the chemical formula SO 4 2. This anion contains four oxygen atoms bonded together around one sulfur atom in an anions’s center in an orderly fashion; two oxygen molecules exist which possess one charge each; this anion can be produced through two methods.
1. Treatment of metal and metal oxide materials using sulfuric acid
2. Oxidizing metal sulfides to sulfites
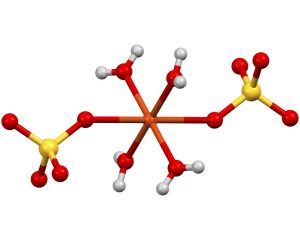
With few exceptions such as strontium sulfate or calcium Sulfate being water-soluble compounds. Furthermore, anionic sulfates could act as ligands to form coordination compounds by joining either one oxygen atom (monodentate an ligand) or two oxygen atoms (bidentate an an ligand).
Difference Between Sulfonate and Sulfate
Sulfonate and Sulfate have distinct chemical structures that differentiate them from each other:
Sulfonate: Sulfonates belong to a functional chemical class called the “sulfonates.” They consist of one sulfur (S) bound directly or indirectly with three oxygen (O), plus an carbon atom (C). Their formulae are R-SO3- where R refers to an organic element; one sulfur may connect directly with carbon while its oxygen bonds remain connected directly with sulfur; their carbon connection varies with each compound and provides for their unique properties and reactive nature.
This structure confers distinct properties on these chemical structures called “sulfonates.” This structure imparts individual properties and reactive characteristics on these reactivity-giving them particular properties and reactive properties when placed alongside their constituent parts – such as their structure gives rise sulfonates their unique properties and reactiveness properties when put under scrutiny!
Sulfate: Sulfate is an elemental group composed of one sulfur atom joined with four oxygen molecules (O). The formula to represent this tetrahedral arrangement of sulfur-oxygen bonds results in two more electrons that balance out its overall charge, known as SO42-. It’s comprised of S and four O atoms held together via double bond connections; one element (S) sits in its center while four Os surround it on all four sides by means of double bonds; its charge balances out overall charge by two.
Sulfonates can be identified through their configuration: one sulfur atom bound to three oxygen and one carbon atoms and an extra sulfur atom connected by four oxygen bonds forming one carbon bond and three bonds between themselves and four oxygen bonds; this configuration and connection of the atoms determines their distinctive properties and behaviors, distinguishing sulfonates and sulfates respectively.
Comparative Charts of Sulfonate and Sulfate
Here’s a chart that compares the main differences between Sulfonate and Sulfate:
| Property | Sulfonate | Sulfate |
|---|---|---|
| Chemical Structure | Sulfur atom is bonded with three oxygen atoms as well as the carbon atom (R-SO3-) | Sulfur atom is bonded to 4 oxygen atoms (SO4^2-) |
| Natural occurrences | Primarily made of synthetic materials, but with a few naturally occurring in marine organisms as well as specific habitats | Minerals naturally abundant in nature, soils, water bodies and living organisms |
| Industrial Applications | Surfactants, detergents and textile processing chemicals pharmaceuticals Personal care products, detergents | Fertilizers, detergents, construction materials |
| Environmental Impact | The majority of biodegradable substances are low toxicity however, potential persistance and accumulation in marine conditions should be monitored | Naturally occurring, however excess levels can cause the eutrophication of aquatic ecosystems and cause harm to them. |
| Health Impacts | Most commonly, they are considered safe to use, certain chemicals may cause irritation to the eyes or skin | It is safe for the majority of people however, excessive levels could result in laxative effects for some individuals. |
| Regulative Aspects | The guidelines and regulations are in place to ensure the safety of handling, usage, as well as discharge | There may be guidelines in place to regulate sulfate levels in drinking water, to ensure the safety of water. |
| Biological Significance | In biological systems, they are less common However, some marine species produce natural Sulfonates | Vital for a variety of biological processes, such as protein synthesis and the development of connective tissues |
This chart of comparison summarizes the primary differences between sulfonate & sulfate in relation to the chemical composition, presence in the natural environment, industrial applications as well as their environmental and the effects on health, regulatory concerns and biological importance.
Composition and Formula
Composition and Formula of Sulfonate: Sulfonates have an R-SO3- composition formula; their organic element represents by R. This chemical compound comprises one sulfur atom (S), connected with three oxygen molecules (O) along with carbon (C), joined to group of organic molecules represented as R (carbon-atom bonds).
A negative charge attached to its ionic form (the S-SO3-) can be neutralised through association with an anionic group such as R, thus mitigating any associated negative charges attached by associated anionic groups associated with R cations groups attached by Cations groups associated with R and vice versa.
Composition and Formula of Sulfates: The most frequently seen formula for sulfate compounds is SO42-, where each ionic bond connects one sulfur atom (S) with four oxygen atoms (O). Two molecules from each oxygen are doubly-bonded to form tetrahedral structures around S.
It has an overall charge Ion of -2 which indicates two extra electrons needed to balance its total charge; similar to its Cation counterpart (sulfonates), many times they form inert compounds which result in inert compounds with zero chemical activity between its constituent parts (S, O).
Sulfonates consist of sulfur bound to three oxygenatoms as well as one carbon atom; SO42 for Sulfates with four oxygen atoms bound. Their composition and arrangement lead to distinct characteristics and behaviors of these ions, offering numerous ways to be classified either way.
Occurrence in Nature
Sulfonates in Nature: Though Sulfonates are typically synthetic compounds found only rarely in nature, some natural Sulfonates exist as natural occurring species like seaweeds produce Sulfonate compounds as part of their defense against predators or to regulate osmotic balance; such naturally-occurring Sulfonates might even form part of marine ecology!
Also, certain soil organisms and fungi produce Sulfonate metabolic products to produce natural Sulfonates which do occur under certain environmental or biological circumstances – though these instances tend to occur less frequently in certain environmental or biological circumstances than others!
Sulfates can be found throughout nature: Sulfates can be found abundantly and widespread presence, from minerals, soils and lakes, and living organisms – making sulfates one of the key ingredients found throughout nature. At present, two common forms of sulfates used commercially include gypsum (calcium dihydrate sulfate) and anhydrite (calcium sulfurate), typically found in sedimentary rocks. Sulfates also exist naturally within seawater where they help control its salinity levels as well as chemical composition.
Within living organisms, sulfates play key roles in biological processes. Sulfate ions integrate with Amino Acids such as methionine and cysteine to produce proteins as well as other biomolecules additionally, they play an integral part in creating connective tissues like cartilage and bone.
Sulfonates are typically synthetic substances; while sulfates occur naturally and can be found in minerals, water bodies, soils and living organisms. Understanding their roles and presence in nature is integral to studying environmental interactions, geological processes and biochemistry of living organisms.
Industrial and Commercial Applications
Industrial and commercial applications of sulfonates:
1. Surfactants and detergents: Sulfonates are frequently utilized as raw material in detergent production due to their excellent cleaning abilities and emulsifying capabilities, enabling them to quickly eliminate grease, dirt, and stains from various surfaces. Sulfonate-based surfactants can be found in household cleaning products like dishwashing detergent, laundry fluid or any number of other dish- or laundry-specific cleaning agents.
2. Chemical Processing for Textiles: Sulfonates can be utilized during textile processing as wetting agents, leveling agents and dye dispersants to enhance dyeing consistency and penetration and dispersion into textile fibers. Sulfonates also assist in washing out unfixed dyes from textiles during washing/finishing cycles for increased dye penetration into their material surfaces.
3. Oilfield Chemicals: Sulfonates are an invaluable addition to drilling liquids and oilfield chemicals in the gas and oil industries as an additive, serving as dispersants, emulsifiers or corrosion inhibitors. Sulfonate-based additives help optimize performance while simultaneously increasing oil recovery while protecting equipment against corrosion.
4. Pharmaceuticals: Sulfonates can be utilized in pharmaceutical formulations as excipients and solubilizing agents to increase bioavailability of less soluble medications while simultaneously improving absorption rate, increasing therapeutic efficacy. Furthermore, Sulfonates are also utilized in producing specific active pharmaceutical components (APIs).
5. Personal Care Products: Sulfonates can be found in many personal care products such as soap shampoos and shower gels as they act as foaming agents emulsifiers and stabilizers for the desired appearance and texture. Sulfonates help create luxurious yet stable lather when cleansing is required.
6. Industrial processes: Sulfonates have applications across numerous industrial processes. For instance, metalworking fluids use them as additives which add flexibility, lubricity and cooling characteristics while water treatment facilities often employ them to combat scaling issues and enhance quality water supplies.
Keep in mind that each application requires using a specific sulfonate compound with unique properties and characteristics, since different sulfonates possess distinct traits and attributes.
Sulfonates play an indispensable role in many commercial and industrial applications, significantly contributing to increased efficiency, effectiveness, and performance of different production processes and goods.
Environmental and Health Considerations
Environmental and health considerations related to sulfonates and sulfates:
Sulfonates:
1. Environmental Impact: These biodegradable materials tend to have low toxicity. But their presence should still be carefully observed because higher concentrations could harm aquatic organisms in fragile environments and could wreak havoc with ecosystem health – thus it’s crucial that their impact be evaluated to minimize damage to both ecosystem and the environment.
2. Regulations and Guidelines: Regulatory bodies like Environmental protection authorities have created rules and guidelines regarding the disposal and use of sulfurates with an aim of limiting their environmental impact and assuring safe disposal practices.
3. Safety and health effects: Sulfonates are generally safe to be used in consumer products, with low human toxicity levels. However, certain compounds within Sulfonates could potentially produce different health-related adverse reactions; certain people could develop eye or skin irritation after coming in contact with certain Sulfonates; thus it’s crucial that safety guidelines and precautions be abided to when dealing with concentrated forms of these sulfates.
Sulfates:
Environmental Impact: At normal concentration levels, sulfates are considered safe chemicals that occur naturally in our environment. However, too much sulfate in water sources may result in eutrophication causing oxygen depletion, damaging ecosystems in the process and ultimately leading to decreased health for residents living nearby.
It is critical that monitoring and managing sulfate levels be maintained so as to mitigate environmental harm caused by excess levels. It’s vitally important for managing our local waters so as to minimize negative environmental consequences from occurring due to this phenomenon and any subsequent environmental issues related to negative environmental issues from occurring.
Regulations and Guidelines: For human health’s sake, regulatory agencies can set standards or set up regulations regarding acceptable concentration levels of sulfate in drinking water to maintain acceptable levels. Using such tools allows regulatory bodies to maintain acceptable sulfate concentration levels.
Health Effects and Safety: Considerations Sulfates in food sources or drinking water is usually safe to ingest; however, individuals suffering from specific medical issues that affect the GI tract, for instance can have laxative-type effects when drinking high amounts of sulfuric compounds such as in sulfates. Therefore it’s crucial that individual’s sensitivities and follow regulations regarding consumption to ensure safety.
Sulfonates and Sulfates are generally Biodegradable compounds with relatively low toxicity however, their environmental and health risk should still be carefully assessed. Sulfates naturally occurring are generally safe but excessive concentrations could pose both ecological and human health impacts; monitoring regulations as well as adhering to safety rules is vital in order to mitigate any negative side-effects related to Sulfonates/Sulfates use.
Conclusion
Sulfonates and Sulfates are two chemical compounds with distinct chemical structures and properties, as well as applications. Sulfonates can be identified by having one sulfur atom bound to three oxygen atoms and carbon, with all four elements linked by covalent bonds. They’re widely used as detergents, surfactants and emulsifiers in industries including textiles, personal care and oilfield chemical; though most synthetic, sulfonates have also found their way into marine environments and specific habitats.
Sulfates consist of sulfur-containing atoms linked by four oxygen atoms and can be found abundantly in nature – including minerals, soils, waters bodies and living things – making up almost 70% of their composition. Sulfates play an essential part of biological processes as well as being utilized by many industries such as fertilizers, detergents and construction materials.