Difference Between Lead Chamber and Contact Process
Sulfuric Acid, an essential component of many industries, is created by two main methods which are the lead chamber method along the contact procedure. The lead chamber method originated in the late 18th century and is in contrast with the more recent contact method that was developed in the 19th century. Understanding the distinctions between these methods is essential due to their effects on efficiency, environmental impacts as well and the quality of sulfuric acid manufacturing.
What is the Lead Chamber Process?
The lead chamber method is a method used in the early days of making sulfuric acid, which dates to the 18th century. It is a chemical reaction in which sulfur dioxide (SO2) produced by burning sulfur reacts with water vapor and oxygen in large chambers that are lined with lead.
This reaction creates sulfur trioxide (SO3) that then reacts with water to create sulfuric acid (H2SO4). This process takes place gradually in lead-lined chambers because of the catalytic properties of the surfaces of lead, allowing the conversion of sulfur dioxide to sulfuric acid over the course of time.
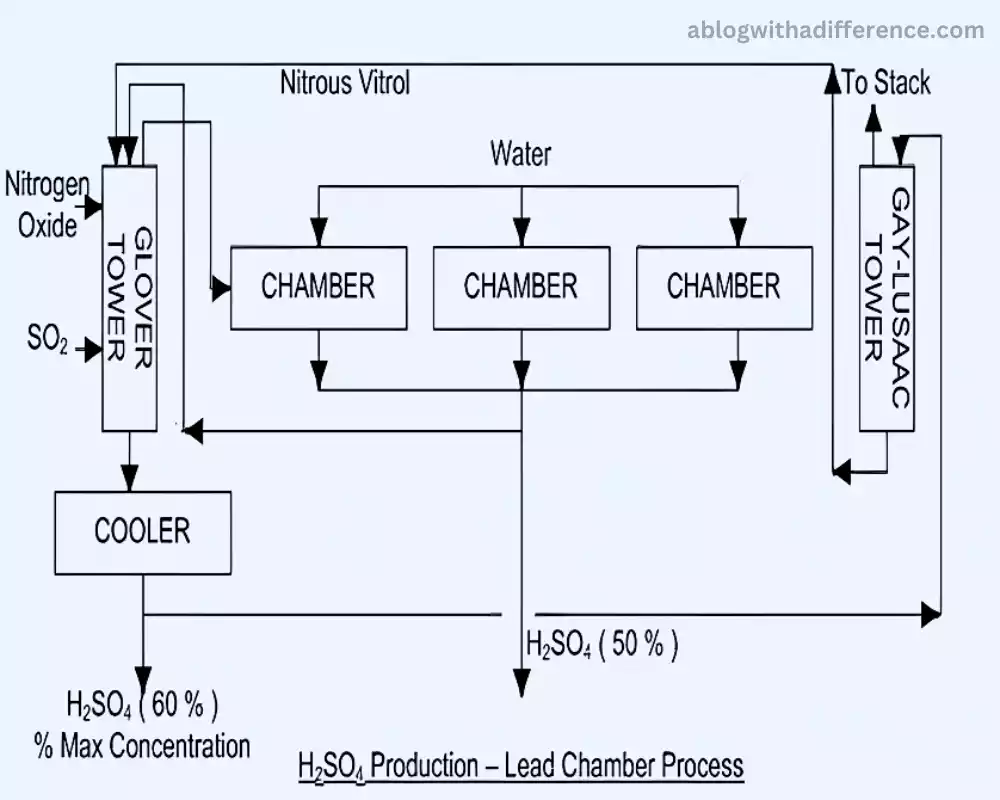
While it is important in the past this process has been less frequent because of health and environmental issues caused by lead contamination. Newer methods, like the contact process, have mostly substituted it due to its improved efficiency and less impact on the environment.
What is the Contact Process?
The contact method is a new industrial technique that is used to produce large quantities of sulfuric acid (H2SO4). This process involves the use of sulfur dioxide (SO2) generated from combustion of sulfur and roasting sulfide minerals is reacted in a reaction with oxygen (O2) in the presence of catalysts to produce sulfur trioxide (SO3). The catalysts that are commonly used are vanadium pentoxide (V2O5) or platinum-rhodium alloys.
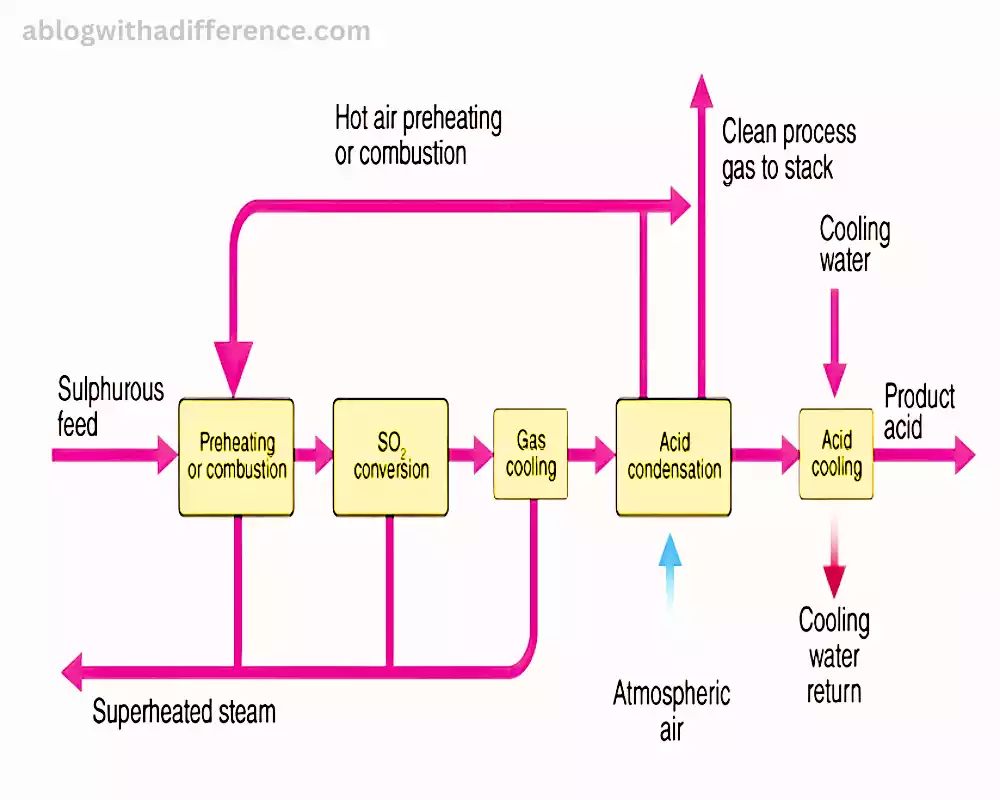
Following the formation of sulfur trioxide this is then taken up into concentrated sulfuric acid. This results in the formation of the oleum (H2S2O7) that is an even more concentrated form that contains sulfuric acid. In the end, oleum is mixed with water to make sulfuric acid that is commercial grade.
The contact method is well-known for its efficiency, speed of production rate, and flexibility which makes it the most popular method for the production of sulfuric acid in all industries. This process greatly reduced environmental impacts compared to earlier methods such as the lead chamber method because of its lower emissions and efficient utilization of resources.
Comparison chart – Lead Chamber and Contact Process
Here’s a concise comparison chart outlining the differences between the Lead Chamber Process and the Contact Process for sulfuric acid production:
| Aspect | Lead Chamber Process | Contact Process |
|---|---|---|
| Historical Development | Originated in the 18th century | Developed in the 19th century |
| Reaction Mechanism | Relies on multiple reactions in lead-lined chambers | Involves catalytic oxidation of sulfur dioxide |
| Catalytic Action | Lead surfaces act as catalysts | Utilizes catalysts like vanadium pentoxide or platinum-rhodium alloys |
| Efficiency | Less efficient due to longer reaction times | More efficient with higher production rates |
| Environmental Impact | Associated with lead contamination, poses environmental and health concerns | Minimizes environmental impact, reduces emissions |
| Current Usage | Less common due to environmental and health concerns | Widely used in modern sulfuric acid production |
This chart summarizes the key differences between the Lead Chamber Process and the Contact Process, highlighting aspects such as their historical background, reaction mechanisms, efficiency, environmental impact, and current usage in sulfuric acid production.
Environmental Impact of the Lead Chamber Process and the Contact Process
The environmental effects of this Lead Chamber Process aren’t so good. Why? It’s all in the lead. This process makes use of lead-lined chambers to conduct chemical reactions to create sulfuric acid. However, lead is detrimental to the environment as well as individuals. It may seep into surroundings, leading to contamination and health hazards, which aren’t good for our planet.
The Contact Process, on the other hand, is more eco-friendly. What’s the reason? This is because the method employs fancy catalysts, such as vanadium pentoxide and platinum-rhodium alloys. These catalysts enable the reaction to occur efficiently, without the need for lead. This means that it creates sulfuric acid, without the threat of lead contamination and is good on the environmental side. Additionally, it helps to reduce harmful emissions, which makes it a healthier option in general.
Safety Measures in Sulfuric Acid Production
Safety measures in sulfuric acid production are crucial due to the potentially hazardous nature of the process. Workers involved in sulfuric acid production need to adhere to strict safety protocols to mitigate risks. Personal protective equipment (PPE) such as goggles, gloves, face shields, and protective clothing is essential to prevent direct contact with sulfuric acid, which is highly corrosive and can cause severe burns upon skin contact.
Adequate ventilation systems are installed to control potentially harmful fumes and ensure a safe working environment. Additionally, proper training and education for workers on handling chemicals, emergency procedures, and spill containment are fundamental safety measures.
Regular inspections, maintenance of equipment, and adherence to safety guidelines and regulations are paramount to prevent accidents and ensure the safety of workers and the surrounding environment.
Industrial Applications of Sulfuric Acid
Sulfuric acid finds widespread use across various industries due to its versatility.
Some key industrial applications include:
- It serves as a fundamental raw material for producing various chemicals like fertilizers, detergents, dyes, and explosives.
- Sulfuric acid is vital in metal processing, including refining metals like copper, zinc, and nickel, as well as in pickling or cleaning steel surfaces.
- It plays a role in refining crude oil, particularly in the production of gasoline additives and removing impurities from fuels.
- It’s utilized in neutralizing alkaline wastewater and for pH control in treatment plants.
- Lead-acid batteries, commonly used in vehicles and backup power systems, rely on sulfuric acid for their electrolyte solution.
- Sulfuric acid is involved in processes such as dyeing, bleaching, and finishing textiles.
- It’s used in paper processing for treating wood pulp and sizing agents.
- These applications showcase the indispensable role of sulfuric acid across multiple industrial sectors due to its corrosive, oxidizing, and dehydrating properties.
Summary
The Lead Chamber Process, with its historical significance, was an early method for sulfuric acid production but faced limitations due to environmental and health concerns associated with lead. In contrast, the Contact Process emerged as a more efficient and environmentally friendly method, utilizing catalysts to produce sulfuric acid without the risks of lead contamination.
The evolution from the lead chamber to the contact process represents a shift towards safer, more efficient industrial practices, emphasizing progress in chemical manufacturing methodologies.