Difference Between 1 Propanol and 2 Propanol
A brief overview of 1 Propanol and 2 Propanol
1.25 Propanol in 2 Propanol Solutions Provided Below is an in-depth review of 1 Propanol = 2. Propanol solutions are an efficient alternative that provides maximum fuel economy at reduced operating costs.
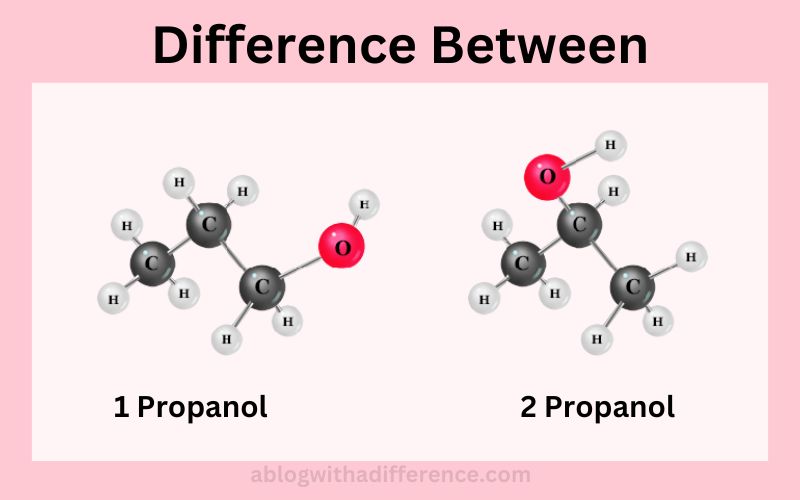
One key difference between one propanol and two propanol lies in their respective chemical makeup: one contains an hydroxyl group attached directly to its carbon chain whereas two propanol has one attached directly to an intermediate carbon atom in its chain.
Propanol 1 and 2 propanol are two isomeric forms of propanol that make up its molecular makeup. Alcohol, propanol is comprised of three carbon atoms linked by carbon chains with one group hydroxyl (-OH) acting as the grouping unit in its structure.
One key distinction between one propanol and two is how its hydroxyl group connects with carbon chains; two differ when their respective carbon chains become integrated together with an additional propanol molecule.
Importance and common uses of 1 Propanol and 2 Propanol
1. Importance and Common Uses of 1 Propanol:
A. Propanol can be considered an indispensable industrial chemical with multiple uses and applications.
B. Solvents like alcohol are widely employed across a range of industries such as paints, coatings, printing inks and adhesives.
C. Used as an intermediate in the production of perfumes, pharmaceuticals and various chemicals.
D. Propanol is widely employed as a cleaning agent within the electronics industry to eliminate residues of flux and other contaminants from PC boards and circuit boards.
E. Used in the production of personal care products, cosmetics products and perfumes.
F. Propanol can be utilized both as an individual fuel and an additive for alcohol-burning engines and stoves.
2. Importance and Common Uses of 2 Propanol:
A. 2 Propanol, also referred to as isopropanol, finds applications across many different industries.
B. Solvents such as mineral spirits and glycol ether are frequently employed to dissolve coatings paints, inks and resins.
C. Propanol is an extremely widely-used cleaner and degreaser that’s widely employed across a range of household, industrial and electronic cleaning applications.
D. Used to disinfect hospitals and maintain personal hygiene.
E. 2 Propanol is an integral component in the manufacturing of pharmaceutical products such as lotions, medicines and topical treatments.
F. Acetone can be utilized as a solvent in extracting and purifying natural products such as essential oils and herb extracts, for use as medicinal uses.
G. 2 Propanol is used as a fuel additive specifically in windshield washer fluid and de-icing products.
2 Propanol plays an integral part in numerous industries due to its properties as a solvent and its ability to dissolve various compounds, with various uses including manufacturing, cleaning and chemical synthesis.
Be mindful that although both alcohols may share common applications, their individual properties and uses could make one better suited than another depending on your specific requirements.
What is 1 Propanol?
This organic compound with the chemical formula C3H8O can best be described as alcohol due to containing one end-connected carbon atom with hydroxyl groups attached.
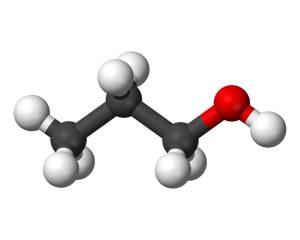
Due to having only a single carbon atom attached, this compound is classified as an alcohol which serves as its primary alcohol type and an isomer made up of propanol; furthermore, it occurs naturally through fermentation processes in very minute amounts.
The molecular mass is 60.09 grams per mole. Additionally, this uncolored liquid features an alcohol-like scent with mild undertones that make for effective usage as pharmaceutical manufacturing solutions and fuel engine applications due to its high octane count.
What is 2 Propanol?
2 propanol (C3H8O), commonly referred to as isopropyl alcohol and with its chemical formula C3H8O is an organic compound composed of carbon, hydrogen and oxygen atoms that ignites easily when mixed together in combustion reactions.
Furthermore, it has an unpleasant odour due to a bonding between its hydroxyl component and carbon chain’s middle carbon atom; hence making this compound secondary alcohol, in addition to being structurally similar to one propanol.
Additionally, it can be mixed with water chloroform ether and ethanol and its viscosity increases significantly at lower temperatures; when exposed to oxygen it may even undergo oxidation to form Acetone.
Two propanol is produced primarily through indirect hydration; when propene is combined with sulfuric acid it forms an ester mixture; hydrolysis then yields isopropyl alcohol as the final product.
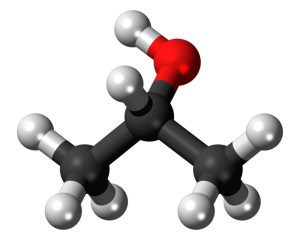
As far as utility in use goes, alcohol can be an efficient solvent to dissolve nonpolar materials like eyeglasses and electronics devices. For this reason, its application includes cleaning applications.
isopropyl alcohol serves as an intermediate chemical component used in the production of isopropyl Acetate and also used to manufacture rubbing alcohol for medicinal use.
Difference Between 1 Propanol and 2 Propanol
1. 1 Propanol (n-Propanol):
Chemical Formula C3H8O
Structural Formula CH3CH2CH2CH2OH
The HH H H C H – C C C C H H OH | | H H H
Description: 1. Propanol which is also referred to as n-Propanol or 1 Propyl Alcohol the group of hydroxyls (-OH) is joined to the carbon atom at the terminal (end of carbon chain).
2. 2. Propanol (Isopropanol):
Chemical Formula C3H8O
Structural Formula: CH3CHOHCH3.
HHHHHH | | | H – C – C – O – H |
Description: 2. Propanol commonly referred to as isopropanol, or isopropyl alcohol the hydroxyl group (-OH) is linked to the carbon atom that is middle (second carbon atom) of the carbon chain.
Each of the 1 Propanol as well as 2 Propanol are alcohols that have three carbon atoms as well as an group of hydroxyls. The primary difference is the location of the hydroxyl atom within the carbon chain. This results in different physical and chemical characteristics for each compound.
Comparative Charts of 1. Propanol with 2 Propanol
Here’s a chart of comparison that highlights the major distinctions among one Propanol in comparison to 2 Propanol:
| Properties | 1 Propanol (n-Propanol) | 2 Propanol (Isopropanol) |
|---|---|---|
| Chemical Formula | C3H8O | C3H8O |
| Structural Formula | CH3CH2CH2OH | CH3CHOHCH3 |
| Boiling Point | 97.2degC | 82.6degC |
| Melting Point | -126.2degC | -89.5degC |
| Solubility | Miscible in water and a variety of organic solvents | Miscible with water as well as numerous organic solvents |
| Density | 0.803 grams/cm3 at 20degC | 0.785 grams/cm3 at 20degC |
| Odor | The distinctive alcohol scent | The distinctive smell of alcohol |
| Appearance | Colorless liquid | Colorless liquid |
| Placement of Hydroxyl Group | Connected to the carbon atom that is terminal (end of carbon chain) | It is attached to the carbon atom in the middle (second carbon atom) of the carbon chain |
| Common Uses | Solvent, an intermediate in chemical synthesis, cosmetics and other personal items for personal care. It can also be used as a fuel additive or additive | Solvent, cleaning agent, disinfectant, pharmaceutical production, fuel additive |
This chart gives a brief outline of the major distinctions between 1 Propanol and 2 Propanol with regard to their chemical physical properties such as solubility, and other common usages.
Physical Properties
1. Physical properties of 1 Propanol (n-Propanol):
Boiling point: 97.2degC
Melting Point: -126.2degC
Solubility: Mixable with water, and a number of organic solvents
Density: 0.803 g/cm3 at 20degC
Odor: A characteristic alcohol-based smell
Appearance: Colorless liquid
2. The Physical Property of Propanol 2 (Isopropanol):
Boiling Point: 82.6degC
Melting Point: -89.5degC
Solubility: Mixable with water, and many organic solvents
Density: 0.785 g/cm3 at 20degC
Odor: Typical alcoholic scent
Appearance: Colorless liquid
Both 1 Propanol and 2 Propanol are colorless liquids that have similar smells. They can be miscible with water as well as many organic solvents. This means that they are able to dissolve easily within these substances.
There are however, significant variations in their boiling points and melt points in addition to small variations in the density. These physical property variations could affect their application in different industries, like cleaners, solvents and pharmaceuticals.
Chemical Properties
1. Chemical Properties of Propanol (n-Propanol):
Oxidation: Propanol can easily undergo the oxidation process and become propanal (propionaldehyde), then later converted to propanoic acid.
Acid-Base Reactions: Some reactions between weak acids and strong bases result in salts being formed; vice versa for weaker acids reacting with stronger bases, producing salts as the products of their reaction.
Esterification: Propanol can undergo esterification reactions that form esters, creating ester-based chemicals.
2. Chemical Characteristics of Isopropanol Propanol 2 (Propanol 2):
Oxidation: 2. Propanol can undergo an oxidation reaction which converts it to Acetone by stripping away two hydrogen atoms from its structure and forms propanone instead.
Acid-Base Reactions: Similar to 1 Propanol. It acts as a weak acid and can form salts when exposed to strong bases.
Esterification: 2. Propanol can undergo esterification reactions with acids to produce esters.
Both 1 Propanol and 2 Propanol share similar chemical properties typical of alcohols both can undergo oxidation reactions that produce various compounds, act like weak acids during acid-base reactions and undergo esterification reactions that create esters.
Chemical reactions depend upon both their conditions of usage and reactions’ products to yield various outcomes; their chemical properties allow for their utilization in various chemical applications or procedures such as manufacturing chemicals, pharmaceutical synthesis or organic chemical reactions.
Toxicity and Safety
1. Toxicity Analysis for Propanol (n-Propanol):
Inhalation: Breathing in toxic vapors may irritate the respiratory tract and result in symptoms like shortness of breath, coughing and chest discomfort. Extended exposure can result in headaches, dizziness and nausea – serious symptoms which should never be ignored!
Skin Contact: Indirect contact with propanol may lead to inflammation, irritation and dryness on the surface of skin. Prolonged or repeated exposure could even result in dermatitis.
Eye Contact: Contact with the eyes can result in redness, irritation and discomfort; in extreme instances it could even result in chemical burns or permanent eye damage.
Consumption: Even one dose of propanol could result in stomach irritation, nausea, vomiting and abdominal discomfort as well as central nervous system depress which could result in confusion, drowsiness and difficulties with coordination.
Chronic Effects: Prolonged exposure to 1 Propanol may damage kidneys and liver over time.
2.Toxicity of 2 Propanol (Isopropanol):
Inhaling: Isopropanol can irritate both the throat and respiratory tract, as well as create breathing issues or coughing fits, at lower doses; higher dosage can result in headaches, dizziness or depression of the central nervous system.
Contact with Skin: Isopropanol can cause skin reddening, itching and dryness on contact; excessive or prolonged exposure could even result in skin irritation.
Eye contact: may cause eye irritation, redness and discomfort; in extreme circumstances chemical exposure could even result in burns as well as irreparable eye damage.
Ingestion: Isopropanol can cause Stomach upset such as nausea and vomiting as well as abdominal discomfort when taken orally in high doses. At higher dosage levels it may trigger Central nervous system depression manifested through symptoms like sleepiness confusion and loss of coordination in some individuals.
Chronic Effects: Extended exposure to isopropanol can have lasting adverse consequences, with potential damage being done to kidneys, liver and the central nervous system.
Safety should always come first when handling one and two Propanol containers; this includes taking precautions like:
For maximum inhalation protection, ensure there is adequate ventilation in work environments to reduce exposure.
Protecting yourself when working with dangerous chemicals requires using protective gear such as gloves, goggles and respirators masks.
Attain skin contact by wearing suitable attire and washing your hands thoroughly after handling products that could contact it.
For safe use in areas that have adequate ventilation or exposure levels are unmonitored properly, respiratory protection may be needed.
Safely store these chemicals in labeled containers away from flames, heat sources or products which might interact negatively.
Make sure that the appropriate disposal and handling procedures comply with local regulations and guidelines.
Consult Safety Data Sheets (SDSs), review any applicable rules, regulations and guidelines related to their safe usage within your specific location or usage situation, as this can help ensure optimal protection.
Applications
1. Application of Propanol (n-Propanol):
Solvent: Propanol has proven its worth as an indispensable solvent across several industries, from coatings and paints to adhesives, inks, cleaners and other cleaning supplies. Due to its broad spectrum solubilization properties it offers great potential in dispersing and solubilizing chemical compounds.
Intermediate: Intermediate is used as an intermediary in the production of perfumes, pharmaceuticals and various other chemicals. It serves both as raw material for chemical synthesis processes as well as acting as a reaction agent in numerous synthetic processes.
Cosmetics and Personal Care: Propanol can be found widely used for personal care and cosmetic applications such as lotions, shampoos, sprays for hair care and perfume production due to its solubility properties and ability to strengthen product stability.
Fuel: Can be utilized both as an independent source and additive in gasoline-burning stoves and engines for specific purposes where other forms of energy sources are limited or nonexistent.
2. Application for 2. Propanol (Isopropanol):
Solvent: 2. Propanol can be widely employed as a solvent in industries like paints coatings and inks, resins or cleaning chemicals, because of its efficiency at dissolving various chemical forms – making it suitable for an array of different purposes and use cases.
Chemical Agents: They’re often employed as cleansing and degreasing agents due to their ability to dissolving oils, greases and other pollutants found in everyday items used for household, industrial and electronic cleaning solutions.
Pharmaceuticals: 2. Propanol is an essential element in the manufacturing of pharmaceutical products, serving both as an extractant and purifier during extraction/purification procedures, and as an integral ingredient when developing lotions/medicines/topical products.
Disinfectant: Given its antimicrobial qualities, 2-Propanol can be employed both as an antiseptic and disinfectant in hospitals such as hospitals, clinics and laboratories for disinfection purposes; specifically on medical equipment surfaces as well as skin.
Fuel additive: Used specifically in windshield washer fluid and de-icing solutions to lower freezing points and improve efficacy, fuel additives are applied as fuel additives to these solutions to increase their effectiveness and lower freezing points.
These applications demonstrate the versatility of propanol as both solvents, cleaners, intermediates and components for various industries including pharmaceuticals, cleaning products, cosmetics and fuel-related items.
Environmental Impact
1. Environmental Impact of 1 Propanol (n-Propanol):
Persistence: 1 Propanol is readily biodegradable, meaning it can be broken down by natural processes in the environment. This characteristic reduces its persistence and potential for long-term environmental accumulation.
Aquatic Toxicity: Propanol is typically considered low toxic in water environments however, high concentrations can harm aquatic organisms including fish and invertebrates. Be cautious to avoid spills into ecosystems that might pollute them further.
Air Pollution: Propanol can contribute to atmospheric pollution by creating ground-level ozone as well as smog production; such ozone production has devastating repercussions for human health as well as quality of air.
Volatility: 1. Propanol has moderate volatility, meaning that its rapid evaporation in the environment pollutes air quality while providing occupational exposure during handling or usage. This process could potentially pollute airways as well as pose risks when handling it at work or handling.
2. Environmental Effects on Propanol (Isopropanol):
Persistence: Isopropanol is biodegradable, meaning its degradation happens naturally within its environment and this reduces its long-term persistence as well as environmental accumulation risks.
Toxicity to Aquatics: Although Isopropanol generally has mild toxic effects in water bodies, excess concentrations could potentially pose harm to aquatic life forms; due to this risk it must be managed carefully so as to avoid spillage or release into bodies of water.
Volatility: Isopropanol is an air pollutant that when released into the environment could contribute to ground-level ozone and smog formation having adverse consequences both on air quality and human health.
Isopropanol’s volatility is moderate. Like 1 Propanol, Isopropanol displays moderate volatility; its quick evaporation could contribute to air pollution as well as present occupational health hazards through handling or use.
Responsible Propanol handling and use is key to mitigating its negative environmental impact, meaning proper storage, disposal, handling procedures as well as adhering to any relevant guidelines or regulations. Implementation of pollution prevention strategies such as containment systems can further help minimize their pollution of the environment.
Conclusion
Propanol (C3H8O) is an organic compound with the chemical formula C3H8O; 2 propanol is also an organic compound with this same composition but which represents its n-isomer.
The primary difference between 1 propanol and 2 propanol lies in their chemical structures; one contains its hydroxyl groups attached at either end of its carbon chain while two contain theirs on carbon atoms in between.